Chondrocyte β-catenin signaling regulates postnatal bone remodeling through modulation of osteoclast formation in a murine model
- PMID: 24431282
- PMCID: PMC3932359
- DOI: 10.1002/art.38195
Chondrocyte β-catenin signaling regulates postnatal bone remodeling through modulation of osteoclast formation in a murine model
Erratum in
-
Incorrect Figure in the Article by Wang et al (Arthritis Rheumatol, January 2014).Arthritis Rheumatol. 2015 Sep;67(9):2465. doi: 10.1002/art.39327. Arthritis Rheumatol. 2015. PMID: 26309052 No abstract available.
Abstract
Objective: To investigate whether β-catenin signaling in chondrocytes regulates osteoclastogenesis, thereby contributing to postnatal bone growth and bone remodeling.
Methods: Mice with conditional knockout (cKO) or conditional activation (cAct) of chondrocyte-specific β-catenin were generated. Changes in bone mass, osteoclast numbers, and osteoblast activity were examined. The mechanisms by which β-catenin signaling in chondrocytes regulates osteoclast formation were determined.
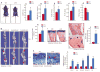
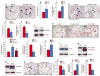
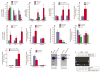
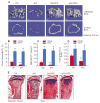
Results: The β-catenin cKO mice developed localized bone loss, whereas cAct mice developed a high bone mass phenotype. Histologic findings suggested that these phenotypes were caused primarily by impaired osteoclast formation, rather than impaired bone formation. Further molecular signaling analyses revealed that β-catenin signaling controlled this process by regulating the expression of the RANKL and osteoprotegerin (OPG) genes in chondrocytes. Activation of β-catenin signaling in chondrocytes suppressed Rankl gene transcription through a glucocorticoid receptor-dependent mechanism. The severe bone loss phenotype observed in β-catenin cKO mice was largely restored by treatment with human recombinant OPG or transgenic overexpression of Opg in chondrocytes.
Conclusion: β-catenin signaling in chondrocytes plays a key role in postnatal bone growth and bone remodeling through its regulation of osteoclast formation.
Copyright © 2014 by the American College of Rheumatology.
Figures


Similar articles
-
Chondrocyte FGFR3 Regulates Bone Mass by Inhibiting Osteogenesis.J Biol Chem. 2016 Nov 25;291(48):24912-24921. doi: 10.1074/jbc.M116.730093. Epub 2016 Oct 11. J Biol Chem. 2016. PMID: 27729453 Free PMC article.
-
Regulatory mechanisms of RANKL presentation to osteoclast precursors.Curr Osteoporos Rep. 2014 Mar;12(1):115-20. doi: 10.1007/s11914-014-0189-0. Curr Osteoporos Rep. 2014. PMID: 24477414 Review.
-
Activation of mTORC1 in B Lymphocytes Promotes Osteoclast Formation via Regulation of β-Catenin and RANKL/OPG.J Bone Miner Res. 2016 Jul;31(7):1320-33. doi: 10.1002/jbmr.2800. Epub 2016 Mar 4. J Bone Miner Res. 2016. PMID: 26825871
-
Naringin increases osteoprotegerin expression in fibroblasts from periprosthetic membrane by the Wnt/β-catenin signaling pathway.J Orthop Surg Res. 2020 Dec 10;15(1):600. doi: 10.1186/s13018-020-02145-z. J Orthop Surg Res. 2020. PMID: 33302980 Free PMC article.
-
Regulatory mechanisms of osteoblast and osteoclast differentiation.Oral Dis. 2002 May;8(3):147-59. doi: 10.1034/j.1601-0825.2002.01829.x. Oral Dis. 2002. PMID: 12108759 Review.
Cited by
-
Osthole enhances the bone mass of senile osteoporosis and stimulates the expression of osteoprotegerin by activating β-catenin signaling.Stem Cell Res Ther. 2021 Feb 27;12(1):154. doi: 10.1186/s13287-021-02228-6. Stem Cell Res Ther. 2021. PMID: 33640026 Free PMC article.
-
Mesenchymal Stem Cells for Cartilage Regeneration of TMJ Osteoarthritis.Stem Cells Int. 2017;2017:5979741. doi: 10.1155/2017/5979741. Epub 2017 Oct 16. Stem Cells Int. 2017. PMID: 29123550 Free PMC article. Review.
-
Osteoclasts and osteoarthritis: Novel intervention targets and therapeutic potentials during aging.Aging Cell. 2024 Apr;23(4):e14092. doi: 10.1111/acel.14092. Epub 2024 Jan 29. Aging Cell. 2024. PMID: 38287696 Free PMC article. Review.
-
Inhibition of Axin1 in osteoblast precursor cells leads to defects in postnatal bone growth through suppressing osteoclast formation.Bone Res. 2020 Aug 12;8:31. doi: 10.1038/s41413-020-0104-5. eCollection 2020. Bone Res. 2020. PMID: 32821442 Free PMC article.
-
Col2CreER(T2), a mouse model for a chondrocyte-specific and inducible gene deletion.Eur Cell Mater. 2014 Oct 23;28:236-45. doi: 10.22203/ecm.v028a16. Eur Cell Mater. 2014. PMID: 25340803 Free PMC article. Review.
References
-
- Adams CS, Shapiro IM. The fate of the terminally differentiated chondrocyte: evidence for microenvironmental regulation of chondrocyte apoptosis. Crit Rev Oral Biol Med. 2002;13:465–73. - PubMed
-
- Gibson G. Active role of chondrocyte apoptosis in endochondral ossification. Microsc Res Tech. 1998;43:191–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

