Glass-like dynamics in the cell and in cellular collectives
- PMID: 24431332
- PMCID: PMC4000035
- DOI: 10.1002/wsbm.1258
Glass-like dynamics in the cell and in cellular collectives
Abstract
Prominent fluctuations, heterogeneity, and cooperativity dominate the dynamics of the cytoskeleton as well as the dynamics of the cellular collective. Such systems are out of equilibrium, disordered, and remain poorly understood. To explain these findings, we consider a unifying mechanistic rubric that imagines these systems as comprising phases of soft condensed matter in proximity to a glass or jamming transition, with associated transitions between solid-like versus liquid-like phases. At the scale of the cytoskeleton, data suggest that intermittent dynamics, kinetic arrest, and dynamic heterogeneity represent mesoscale features of glassy protein-protein interactions that link underlying biochemical events to integrative cellular behaviors such as crawling, contraction, and remodeling. At the scale of the multicellular collective, jamming has the potential to unify diverse biological factors that previously had been considered mostly as acting separately and independently. Although a quantitative relationship between intra- and intercellular dynamics is still lacking, glassy dynamics and jamming offer insights linking the mechanobiology of cell to human physiology and pathophysiology.
© 2014 Wiley Periodicals, Inc.
Figures

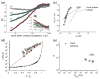
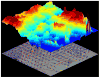
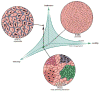
References
-
- Kennedy D, Norman C. What don't we know? Science. 2005;309:75. - PubMed
-
- Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. Scaling the microrheology of living cells. Physical Review Letters. 2001;87:148102. - PubMed
-
- Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Taback NA, Millet EJ, Fredberg JJ. Time scale and other invariants of integrative mechanical behavior in living cells. Physical Review E. 2003;68:041914. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

