Certolizumab pegol in rheumatoid arthritis patients with low to moderate activity: the CERTAIN double-blind, randomised, placebo-controlled trial
- PMID: 24431394
- PMCID: PMC4392224
- DOI: 10.1136/annrheumdis-2013-204632
Certolizumab pegol in rheumatoid arthritis patients with low to moderate activity: the CERTAIN double-blind, randomised, placebo-controlled trial
Abstract
Objectives: This 52-week, randomised, double-blind phase IIIb study assessed efficacy and safety of certolizumab pegol (CZP) as add-on therapy to non-biologic disease-modifying antirheumatic drugs (DMARDs) in rheumatoid arthritis (RA) patients with low to moderate disease activity, and stopping therapy in patients in sustained remission.
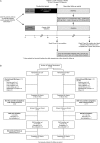
Methods: Patients were randomised 1:1 to CZP (400 mg at weeks 0, 2 and 4, then 200 mg every 2 weeks) or placebo (every 2 weeks) plus current non-biologic DMARDs. At week 24, patients who achieved the primary endpoint of Clinical Disease Activity Index (CDAI) remission at both weeks 20 and 24 stopped study treatment and continued in the study until week 52.
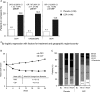
Results: Of 194 patients (CZP=96; placebo=98), >90% had moderate disease activity at baseline. Significantly more CZP patients met the primary endpoint than placebo patients (week 20 and 24 CDAI remission rates: 18.8% vs 6.1%; p≤0.05). At week 24, 63.0% vs 29.7% of CZP versus placebo patients (p<0.001) achieved LDA. Disease activity score (ESR) based on 28-joint count and Simplified Disease Activity Index remission rates were also significantly higher with CZP versus placebo (19.8% vs 3.1%; p≤0.01 and 14.6% vs 4.1%; p≤0.05). CZP patients reported improvements in physical function versus placebo (mean Health Assessment Questionnaire-Disability-Index change from baseline: CZP, -0.25 vs placebo, -0.03; p≤0.01). During the period following withdrawal of CZP or placebo, only 3/17 prior CZP patients and 2/6 prior placebo patients maintained CDAI remission until week 52, but CZP reinstitution allowed renewed improvement. Adverse and serious adverse event rates were comparable between CZP and placebo groups.
Conclusions: Addition of CZP to non-biologic DMARDs is an effective treatment in RA patients with predominantly moderate disease activity, allowing low-disease activity or remission to be reached in a majority of the patients. However, the data suggest that CZP cannot be withdrawn in patients achieving remission.
Trial registration number: NCT00674362.
Keywords: Anti-TNF; DMARDs (biologic); Disease Activity; Rheumatoid Arthritis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

References
-
- Montag K, Gingold M, Boers A, et al. Disease-modifying anti-rheumatic drug usage, prescribing patterns and disease activity in rheumatoid arthritis patients in community-based practice. Intern Med J 2011;41:450–5. - PubMed
-
- Conaghan PG, Hensor EM, Keenan AM, et al. Persistently moderate DAS-28 is not benign: loss of function occurs in early RA despite step-up DMARD therapy. Rheumatology (Oxford) 2010;49:1894–9. - PubMed
-
- Kiely P, Walsh D, Williams R, et al. Outcome in rheumatoid arthritis patients with continued conventional therapy for moderate disease activity—the early RA network (ERAN). Rheumatology (Oxford) 2011;50:926–31. - PubMed
-
- Mierau M, Schoels M, Gonda G, et al. Assessing remission in clinical practice. Rheumatology (Oxford) 2007;46:975–9. - PubMed
-
- Macedo A, Oakley S, Gullick N, et al. An examination of work instability, functional impairment, and disease activity in employed patients with rheumatoid arthritis. J Rheumatol 2009;36:225–30. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

