Neuronal expression of GalNAc transferase is sufficient to prevent the age-related neurodegenerative phenotype of complex ganglioside-deficient mice
- PMID: 24431446
- PMCID: PMC3891965
- DOI: 10.1523/JNEUROSCI.3996-13.2014
Neuronal expression of GalNAc transferase is sufficient to prevent the age-related neurodegenerative phenotype of complex ganglioside-deficient mice
Abstract
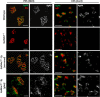
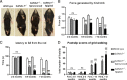
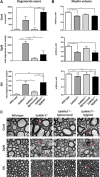
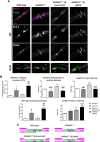
Gangliosides are widely expressed sialylated glycosphingolipids with multifunctional properties in different cell types and organs. In the nervous system, they are highly enriched in both glial and neuronal membranes. Mice lacking complex gangliosides attributable to targeted ablation of the B4galnt1 gene that encodes β-1,4-N-acetylegalactosaminyltransferase 1 (GalNAc-transferase; GalNAcT(-/-)) develop normally before exhibiting an age-dependent neurodegenerative phenotype characterized by marked behavioral abnormalities, central and peripheral axonal degeneration, reduced myelin volume, and loss of axo-glial junction integrity. The cell biological substrates underlying this neurodegeneration and the relative contribution of either glial or neuronal gangliosides to the process are unknown. To address this, we generated neuron-specific and glial-specific GalNAcT rescue mice crossed on the global GalNAcT(-/-) background [GalNAcT(-/-)-Tg(neuronal) and GalNAcT(-/-)-Tg(glial)] and analyzed their behavioral, morphological, and electrophysiological phenotype. Complex gangliosides, as assessed by thin-layer chromatography, mass spectrometry, GalNAcT enzyme activity, and anti-ganglioside antibody (AgAb) immunohistology, were restored in both neuronal and glial GalNAcT rescue mice. Behaviorally, GalNAcT(-/-)-Tg(neuronal) retained a normal "wild-type" (WT) phenotype throughout life, whereas GalNAcT(-/-)-Tg(glial) resembled GalNAcT(-/-) mice, exhibiting progressive tremor, weakness, and ataxia with aging. Quantitative electron microscopy demonstrated that GalNAcT(-/-) and GalNAcT(-/-)-Tg(glial) nerves had significantly increased rates of axon degeneration and reduced myelin volume, whereas GalNAcT(-/-)-Tg(neuronal) and WT appeared normal. The increased invasion of the paranode with juxtaparanodal Kv1.1, characteristically seen in GalNAcT(-/-) and attributed to a breakdown of the axo-glial junction, was normalized in GalNAcT(-/-)-Tg(neuronal) but remained present in GalNAcT(-/-)-Tg(glial) mice. These results indicate that neuronal rather than glial gangliosides are critical to the age-related maintenance of nervous system integrity.
Keywords: ganglioside; glycosyltransferase; neurodegeneration; transgenic.
Figures


Similar articles
-
Neuronally expressed a-series gangliosides are sufficient to prevent the lethal age-dependent phenotype in GM3-only expressing mice.J Neurochem. 2021 Jul;158(2):217-232. doi: 10.1111/jnc.15365. Epub 2021 May 12. J Neurochem. 2021. PMID: 33864399
-
Glial Sulfatides and Neuronal Complex Gangliosides Are Functionally Interdependent in Maintaining Myelinating Axon Integrity.J Neurosci. 2019 Jan 2;39(1):63-77. doi: 10.1523/JNEUROSCI.2095-18.2018. Epub 2018 Nov 16. J Neurosci. 2019. PMID: 30446529 Free PMC article.
-
Ganglioside deficiency causes inflammation and neurodegeneration via the activation of complement system in the spinal cord.J Neuroinflammation. 2014 Mar 28;11:61. doi: 10.1186/1742-2094-11-61. J Neuroinflammation. 2014. PMID: 24673754 Free PMC article.
-
Epigenetic regulation of ganglioside expression in neural stem cells and neuronal cells.Glycoconj J. 2017 Dec;34(6):749-756. doi: 10.1007/s10719-016-9719-6. Epub 2016 Aug 19. Glycoconj J. 2017. PMID: 27540730 Free PMC article. Review.
-
Glycolipids: Essential regulator of neuro-inflammation, metabolism and gliomagenesis.Biochim Biophys Acta Gen Subj. 2017 Oct;1861(10):2479-2484. doi: 10.1016/j.bbagen.2017.06.007. Epub 2017 Jun 7. Biochim Biophys Acta Gen Subj. 2017. PMID: 28602513 Review.
Cited by
-
C1q-targeted inhibition of the classical complement pathway prevents injury in a novel mouse model of acute motor axonal neuropathy.Acta Neuropathol Commun. 2016 Mar 2;4:23. doi: 10.1186/s40478-016-0291-x. Acta Neuropathol Commun. 2016. PMID: 26936605 Free PMC article.
-
Schwann cell nodal membrane disruption triggers bystander axonal degeneration in a Guillain-Barré syndrome mouse model.J Clin Invest. 2022 Jul 15;132(14):e158524. doi: 10.1172/JCI158524. J Clin Invest. 2022. PMID: 35671105 Free PMC article.
-
Sialylation regulates brain structure and function.FASEB J. 2015 Jul;29(7):3040-53. doi: 10.1096/fj.15-270983. Epub 2015 Apr 6. FASEB J. 2015. PMID: 25846372 Free PMC article.
-
Sialylation and Galectin-3 in Microglia-Mediated Neuroinflammation and Neurodegeneration.Front Cell Neurosci. 2020 Jun 9;14:162. doi: 10.3389/fncel.2020.00162. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32581723 Free PMC article. Review.
-
Sialic Acid Is Required for Neuronal Inhibition by Soluble MAG but not for Membrane Bound MAG.Front Mol Neurosci. 2016 Apr 1;9:21. doi: 10.3389/fnmol.2016.00021. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27065798 Free PMC article.
References
-
- Boffey J, Odaka M, Nicoll D, Wagner ER, Townson K, Bowes T, Conner J, Furukawa K, Willison HJ. Characterisation of the immunoglobulin variable region gene usage encoding the murine anti-ganglioside antibody repertoire. J Neuroimmunol. 2005;165:92–103. doi: 10.1016/j.jneuroim.2005.04.011. - DOI - PubMed
-
- Boukhris A, Schule R, Loureiro JL, Lourenço CM, Mundwiller E, Gonzalez MA, Charles P, Gauthier J, Rekik I, Acosta Lebrigio RF, Gaussen M, Speziani F, Ferbert A, Feki I, Caballero-Oteyza A, Dionne-Laporte A, Amri M, Noreau A, Forlani S, et al. Alteration of ganglioside biosynthesis responsible for complex hereditary spastic paraplegia. Am J Hum Genet. 2013;93:118–123. doi: 10.1016/j.ajhg.2013.05.006. - DOI - PMC - PubMed
-
- Bowes T, Wagner ER, Boffey J, Nicholl D, Cochrane L, Benboubetra M, Conner J, Furukawa K, Furukawa K, Willison HJ. Tolerance to self gangliosides is the major factor restricting the antibody response to lipopolysaccharide core oligosaccharides in Campylobacter jejuni strains associated with Guillain-Barre syndrome. Infect Immun. 2002;70:5008–5018. doi: 10.1128/IAI.70.9.5008-5018.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
