Efficacy and safety of duloxetine 60 mg once daily in major depressive disorder: a review with expert commentary
- PMID: 24432034
- PMCID: PMC3884746
- DOI: 10.7573/dic.212245
Efficacy and safety of duloxetine 60 mg once daily in major depressive disorder: a review with expert commentary
Abstract
Objective: Major depressive disorder (MDD) is a significant public health concern and challenges health care providers to intervene with appropriate treatment. This article provides an overview of efficacy and safety information for duloxetine 60 mg/day in the treatment of MDD, including its effect on painful physical symptoms (PPS).
Design: A literature search was conducted for articles and pooled analyses reporting information regarding the use of duloxetine 60 mg/day in placebo-controlled trials.
Setting: Placebo-controlled, active-comparator, short- and long-term studies were reviewed.
Participants: Adult (≥18 years) patients with MDD.
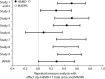
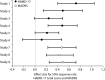
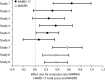
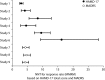
Measurements: Effect sizes for continuous outcome (change from baseline to endpoint) and categorical outcome (response and remission rates) were calculated using the primary measures of 17-item Hamilton Rating Scale for Depression (HAMD-17) or Montgomery-Åsberg Depression Rating Scale (MADRS) total score. The Brief Pain Inventory and Visual Analogue Scales were used to assess improvements in PPS. Glass estimation method was used to calculate effect sizes, and numbers needed to treat (NNT) were calculated based on HAMD-17 and MADRS total scores for remission and response rates. Safety data were examined via the incidence of treatment-emergent adverse events and by mean changes in vital-sign measures.
Results: Treatment with duloxetine was associated with small-to-moderate effect sizes in the range of 0.12 to 0.72 for response rate and 0.07 to 0.65 for remission rate. NNTs were in the range of 3 to 16 for response and 3 to 29 for remission. Statistically significant improvements (p≤0.05) were observed in duloxetine-treated patients compared to placebo-treated patients in PPS and quality of life. The safety profile of the 60-mg dose was consistent with duloxetine labeling, with the most commonly observed significant adverse events being nausea, dry mouth, diarrhea, dizziness, constipation, fatigue, and decreased appetite.
Conclusion: These results reinforce the efficacy and tolerability of duloxetine 60 mg/day as an effective short- and long-term treatment for adults with MDD. The evidence of the independent analgesic effect of duloxetine 60 mg/day supports its use as a treatment for patients with PPS associated with depression. This review is limited by the fact that it included randomized clinical trials with different study designs. Furthermore, data from randomized controlled trials may not generalize well to real clinical practice.
Keywords: duloxetine; effect size; major depressive disorder; painful physical symptoms; quality of life; safety and tolerability.
Figures
Similar articles
-
Duloxetine in the acute and long-term treatment of major depressive disorder: a placebo- and paroxetine-controlled trial.Eur Neuropsychopharmacol. 2004 Dec;14(6):457-70. doi: 10.1016/j.euroneuro.2004.01.002. Eur Neuropsychopharmacol. 2004. PMID: 15589385 Clinical Trial.
-
A randomized placebo-controlled trial of duloxetine in patients with major depressive disorder and associated painful physical symptoms.Curr Med Res Opin. 2011 Oct;27(10):1849-58. doi: 10.1185/03007995.2011.609539. Epub 2011 Aug 12. Curr Med Res Opin. 2011. PMID: 21838411 Clinical Trial.
-
Efficacy and safety of duloxetine 60 mg once daily in the treatment of pain in patients with major depressive disorder and at least moderate pain of unknown etiology: a randomized controlled trial.J Clin Psychiatry. 2007 Nov;68(11):1707-16. doi: 10.4088/jcp.v68n1110. J Clin Psychiatry. 2007. PMID: 18052564 Clinical Trial.
-
Duloxetine: a review of its use in the treatment of generalized anxiety disorder.CNS Drugs. 2009;23(6):523-41. doi: 10.2165/00023210-200923060-00006. CNS Drugs. 2009. PMID: 19480470 Review.
-
Efficacy, safety and tolerability of duloxetine 60 mg once daily in major depression.Curr Med Res Opin. 2005 Mar;21(3):345-56. doi: 10.1185/030079905X30680. Curr Med Res Opin. 2005. PMID: 15811202 Review.
Cited by
-
Small Molecule Agonists of Cell Adhesion Molecule L1 Mimic L1 Functions In Vivo.Mol Neurobiol. 2016 Sep;53(7):4461-83. doi: 10.1007/s12035-015-9352-6. Epub 2015 Aug 8. Mol Neurobiol. 2016. PMID: 26253722
-
A Randomized, Double-Blind, Placebo- and Active Comparator-Controlled Phase I Study of Analgesic/Antihyperalgesic Properties of ASP8477, a Fatty Acid Amide Hydrolase Inhibitor, in Healthy Female Subjects.Pain Med. 2018 Jun 1;19(6):1206-1218. doi: 10.1093/pm/pnx281. Pain Med. 2018. PMID: 29228247 Free PMC article. Clinical Trial.
-
Major Depressive Disorder and Kappa Opioid Receptor Antagonists.Transl Perioper Pain Med. 2016;1(2):4-16. Transl Perioper Pain Med. 2016. PMID: 27213169 Free PMC article.
-
Treatment of major depressive disorders with generic duloxetine and paroxetine: a multi-centered, double-blind, double-dummy, randomized controlled clinical trial.Shanghai Arch Psychiatry. 2015 Aug 25;27(4):228-36. doi: 10.11919/j.issn.1002-0829.215064. Shanghai Arch Psychiatry. 2015. PMID: 26549959 Free PMC article.
-
Randomized controlled trials of serotonin-norepinephrine reuptake inhibitor in treating major depressive disorder in children and adolescents: a meta-analysis of efficacy and acceptability.Braz J Med Biol Res. 2016 May 24;49(6):e4806. doi: 10.1590/1414-431X20164806. Braz J Med Biol Res. 2016. PMID: 27240293 Free PMC article. Review.
References
-
- Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Disord. 2002;72:227–36. - PubMed
-
- Simon GE, Chisholm D, Treglia M, Bushnell D, LIDO Group Course of depression, health services costs, and work productivity in an international primary care study. Gen Hosp Psychiatry. 2002;24:328–35. - PubMed
-
- World Health Organization The Global Burden of Disease: 2004 Update. http://www.who.int/healthinfo/global_burden_disease/2004_report_update/e.... Accessed December 12, 2012.
-
- World Health Organization Depression. http://www.who.int/mental_health/management/depression/en/. Accessed December 12, 2012.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

