The effects of botulinum toxin injection frequency on calf muscle growth in young children with spastic cerebral palsy: a 12-month prospective study
- PMID: 24432106
- PMCID: PMC3838523
- DOI: 10.1007/s11832-013-0503-x
The effects of botulinum toxin injection frequency on calf muscle growth in young children with spastic cerebral palsy: a 12-month prospective study
Abstract
Purpose: This study was a 12-month prospective investigation of changes in the medial gastrocnemius (MG) muscle morphology in children aged 2-5 years with spastic cerebral palsy (CP) who had received no previous intramuscular injections of botulinum neurotoxin type-A (BoNT-A) and were randomised to receive either single or multiple (three) BoNT-A injections to the gastrocsoleus. MG morphological changes were compared to age-matched typically developing (TD) peers.
Methods: Thirteen children with spastic CP with a mean age of 45 (15) months and 18 TD children with a mean age of 48 (14) months participated in the study. The principal outcome measures were MG muscle volume, fascicle length, pennation angle and physiological cross-sectional area (PCSA), which were obtained using 2D and 3D ultrasound.
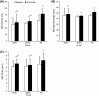
Results: The single and multiple injection frequency groups significantly increased MG muscle volume at 12 months relative to the baseline by 13 and 15 %, respectively. There were no significant differences in the MG muscle volume 28.5 (12.3) versus 30.3 (3.8) ml, fascicle length 48.0 (10.4) versus 44.8 (1.2) mm or PCSA 7.0 (1.2) versus 6.6 (1.7) cm(2) between the single and multiple injection groups, respectively, at 12 months follow-up. The change in MG muscle volume in the single and multiple injection groups was significantly lower than the TD peers by 66 and 60 %, respectively.
Interpretation: In young children with spastic CP, naive to BoNT-A treatment, MG muscle growth over 12 months does not appear to be influenced by intramuscular BoNT-A injection frequency. However, MG muscle growth in the spastic CP groups was significantly lower than the age-matched TD peers. It is unclear whether this is an effect of intramuscular BoNT-A injections or reduced growth rates in children with spastic CP in general. Controlled investigations and longitudinal studies with multiple measurement time points are required in order to determine the influence of BoNT-A treatment on muscle physiological and mechanical growth factors in young children with spastic CP.
Keywords: Botulinum neurotoxin type-A; Cerebral palsy; Medial gastrocnemius; Muscle volume; Physiological cross-sectional area; Ultrasound.
Figures
Similar articles
-
Medial gastrocnemius muscle volume and fascicle length in children aged 2 to 5 years with cerebral palsy.Dev Med Child Neurol. 2011 Jun;53(6):543-8. doi: 10.1111/j.1469-8749.2011.03913.x. Epub 2011 Apr 20. Dev Med Child Neurol. 2011. PMID: 21506995
-
Medial gastrocnemius volume and echo-intensity after botulinum neurotoxin A interventions in children with spastic cerebral palsy.Dev Med Child Neurol. 2019 Jul;61(7):783-790. doi: 10.1111/dmcn.14056. Epub 2018 Oct 15. Dev Med Child Neurol. 2019. PMID: 30320442
-
Reduced Cross-Sectional Muscle Growth Six Months after Botulinum Toxin Type-A Injection in Children with Spastic Cerebral Palsy.Toxins (Basel). 2022 Feb 14;14(2):139. doi: 10.3390/toxins14020139. Toxins (Basel). 2022. PMID: 35202166 Free PMC article.
-
Effectiveness of botulinum toxin A for upper and lower limb spasticity in children with cerebral palsy: a summary of evidence.J Neural Transm (Vienna). 2009 Mar;116(3):319-31. doi: 10.1007/s00702-008-0175-8. Epub 2009 Jan 14. J Neural Transm (Vienna). 2009. PMID: 19142573 Review.
-
Peri-operative Botulinum Neurotoxin injection to improve outcomes of surgeries on spastic limbs: A systematic review.Toxicon. 2020 Dec;188:48-54. doi: 10.1016/j.toxicon.2020.10.005. Epub 2020 Oct 10. Toxicon. 2020. PMID: 33045238
Cited by
-
Reliability of 3D freehand ultrasound to assess lower limb muscles in children with spastic cerebral palsy and typical development.J Anat. 2023 Jun;242(6):986-1002. doi: 10.1111/joa.13839. Epub 2023 Feb 20. J Anat. 2023. PMID: 36807218 Free PMC article.
-
Botulinum toxin injection causes hyper-reflexia and increased muscle stiffness of the triceps surae muscle in the rat.J Neurophysiol. 2016 Dec 1;116(6):2615-2623. doi: 10.1152/jn.00452.2016. Epub 2016 Sep 14. J Neurophysiol. 2016. PMID: 27628204 Free PMC article.
-
Comment on: "Botulinum Toxin in the Management of Children with Cerebral Palsy".Paediatr Drugs. 2019 Dec;21(6):493-495. doi: 10.1007/s40272-019-00358-2. Paediatr Drugs. 2019. PMID: 31583614 No abstract available.
-
Over 25 Years of Pediatric Botulinum Toxin Treatments: What Have We Learned from Injection Techniques, Doses, Dilutions, and Recovery of Repeated Injections?Toxins (Basel). 2020 Jul 6;12(7):440. doi: 10.3390/toxins12070440. Toxins (Basel). 2020. PMID: 32640636 Free PMC article. Review.
-
Botulinum Toxin Intervention in Cerebral Palsy-Induced Spasticity Management: Projected and Contradictory Effects on Skeletal Muscles.Toxins (Basel). 2022 Nov 8;14(11):772. doi: 10.3390/toxins14110772. Toxins (Basel). 2022. PMID: 36356022 Free PMC article. Review.
References
-
- Love SC, Novak I, Kentish M, Desloovere K, Heinen F, Molenaers G, O’Flaherty S, Graham HK, Cerebral Palsy Institute Botulinum toxin assessment, intervention and after-care for lower limb spasticity in children with cerebral palsy: international consensus statement. Eur J Neurol. 2010;17:9–37. doi: 10.1111/j.1468-1331.2010.03126.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

