Fluorescent labeling and modification of proteins
- PMID: 24432126
- PMCID: PMC3691395
- DOI: 10.1007/s12154-013-0094-5
Fluorescent labeling and modification of proteins
Abstract
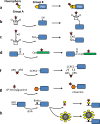
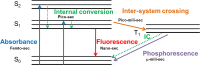
This review provides an outline for fluorescent labeling of proteins. Fluorescent assays are very diverse providing the most sensitive and robust methods for observing biological processes. Here, different types of labels and methods of attachment are discussed in combination with their fluorescent properties. The advantages and disadvantages of these different methods are highlighted, allowing the careful selection for different applications, ranging from ensemble spectroscopy assays through to single-molecule measurements.
Keywords: Fluorescence; Fluorescent proteins; Fluorophores; Quantum dots; Single molecule.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

