Alternative synthetic tools to phospho-specific antibodies for phosphoproteome analysis: progress and prospects
- PMID: 24432133
- PMCID: PMC3787206
- DOI: 10.1007/s12154-013-0100-y
Alternative synthetic tools to phospho-specific antibodies for phosphoproteome analysis: progress and prospects
Abstract
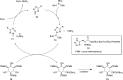
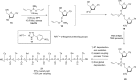
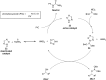
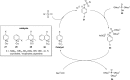
Signal transduction cascades in living systems are often controlled via post-translational phosphorylation and dephosphorylation of proteins. These processes are catalyzed in vivo by kinase and phosphatase enzymes, which consequently play an important role in many disease states, including cancer and immune system disorders. Current techniques for studying the phosphoproteome (isotopic labeling, chromatographic techniques, and phosphospecific antibodies), although undoubtedly very powerful, have yet to provide a generic tool for phosphoproteomic analysis despite the widespread utility such a technique would have. The use of small molecule organic catalysts that can promote selective phosphate esterification could provide a useful alternative to current state-of-the-art techniques for use in, e.g., the labeling and pull-down of phosphorylated proteins. This report reviews current techniques used for phosphoproteomic analysis and the recent use of small molecule peptide-based catalysts in phosphorylation reactions, indicating possible future applications for this type of catalyst as synthetic alternatives to phosphospecific antibodies for phosphoproteome analysis.
Keywords: Kinase mimetic; Kinase mimic; Nucleophilic catalysis; Phosphoproteome; Phosphorylation; Phosphospecific antibody.
Figures

Similar articles
-
The developmental phosphoproteome of Haemonchus contortus.J Proteomics. 2020 Feb 20;213:103615. doi: 10.1016/j.jprot.2019.103615. Epub 2019 Dec 14. J Proteomics. 2020. PMID: 31846766
-
Chemical Catalysis Intervening to Histone Epigenetics.Acc Chem Res. 2021 May 4;54(9):2313-2322. doi: 10.1021/acs.accounts.1c00144. Epub 2021 Apr 13. Acc Chem Res. 2021. PMID: 33847478 Review.
-
Profiling the global tyrosine phosphorylation state.Mol Cell Proteomics. 2003 Apr;2(4):215-33. doi: 10.1074/mcp.R300002-MCP200. Epub 2003 May 17. Mol Cell Proteomics. 2003. PMID: 12754303 Review.
-
Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium.Biochemistry. 2006 Feb 28;45(8):2524-36. doi: 10.1021/bi052475e. Biochemistry. 2006. PMID: 16489745 Free PMC article.
-
Current chemical biology tools for studying protein phosphorylation and dephosphorylation.Chemistry. 2012 Jan 2;18(1):28-39. doi: 10.1002/chem.201103206. Epub 2011 Dec 9. Chemistry. 2012. PMID: 22161995
References
-
- Martic S, Kraatz HB. Chem Sci. 2013;4:42–59. doi: 10.1039/c2sc20846f. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

