Lysine overproducing Corynebacterium glutamicum is characterized by a robust linear combination of two optimal phenotypic states
- PMID: 24432142
- PMCID: PMC3641285
- DOI: 10.1007/s11693-013-9107-5
Lysine overproducing Corynebacterium glutamicum is characterized by a robust linear combination of two optimal phenotypic states
Abstract
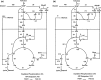
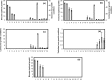
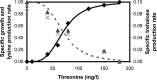
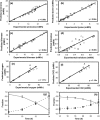
A homoserine auxotroph strain of Corynebacterium glutamicum accumulates storage compound trehalose with lysine when limited by growth. Industrially lysine is produced from C. glutamicum through aspartate biosynthetic pathway, where enzymatic activity of aspartate kinase is allosterically controlled by the concerted feedback inhibition of threonine plus lysine. Ample threonine in the medium supports growth and inhibits lysine production (phenotype-I) and its complete absence leads to inhibition of growth in addition to accumulating lysine and trehalose (phenotype-II). In this work, we demonstrate that as threonine concentration becomes limiting, metabolic state of the cell shifts from maximizing growth (phenotype-I) to maximizing trehalose phenotype (phenotype-II) in a highly sensitive manner (with a Hill coefficient of 4). Trehalose formation was linked to lysine production through stoichiometry of the network. The study demonstrated that the net flux of the population was a linear combination of the two optimal phenotypic states, requiring only two experimental measurements to evaluate the flux distribution. The property of linear combination of two extreme phenotypes was robust for various medium conditions including varying batch time, initial glucose concentrations and medium osmolality.
Keywords: Corynebacterium glutamicum; Elementary modes; Lysine; Optimal phenotypic state; Osmotic stress.
Figures


Similar articles
-
Phenotypic characterization of Corynebacterium glutamicum under osmotic stress conditions using elementary mode analysis.J Ind Microbiol Biotechnol. 2011 Sep;38(9):1345-57. doi: 10.1007/s10295-010-0918-z. Epub 2010 Dec 5. J Ind Microbiol Biotechnol. 2011. PMID: 21132515
-
Molecular aspects of lysine, threonine, and isoleucine biosynthesis in Corynebacterium glutamicum.Antonie Van Leeuwenhoek. 1993-1994;64(2):145-63. doi: 10.1007/BF00873024. Antonie Van Leeuwenhoek. 1993. PMID: 8092856 Review.
-
Construction of L-lysine-, L-threonine-, and L-isoleucine-overproducing strains of Corynebacterium glutamicum.Ann N Y Acad Sci. 1996 May 15;782:25-39. doi: 10.1111/j.1749-6632.1996.tb40544.x. Ann N Y Acad Sci. 1996. PMID: 8659901 Review.
-
Osmotic stress response: quantification of cell maintenance and metabolic fluxes in a lysine-overproducing strain of Corynebacterium glutamicum.Appl Environ Microbiol. 2004 Jul;70(7):4222-9. doi: 10.1128/AEM.70.7.4222-4229.2004. Appl Environ Microbiol. 2004. PMID: 15240305 Free PMC article.
-
Phenotypic characterization of Corynebacterium glutamicum using elementary modes towards synthesis of amino acids.Syst Synth Biol. 2010 Dec;4(4):281-91. doi: 10.1007/s11693-011-9073-8. Epub 2011 Feb 22. Syst Synth Biol. 2010. PMID: 22132055 Free PMC article.
Cited by
-
Omics-driven identification and elimination of valerolactam catabolism in Pseudomonas putida KT2440 for increased product titer.Metab Eng Commun. 2019 Aug 10;9:e00098. doi: 10.1016/j.mec.2019.e00098. eCollection 2019 Dec. Metab Eng Commun. 2019. PMID: 31720214 Free PMC article.
References
-
- Chen N, Du J, Liu H, Xu QY. Elementary mode analysis and metabolic flux analysis of l-glutamate biosynthesis by Corynebacterium glutamicum. Ann Microbiol. 2009;59(2):317–322. doi: 10.1007/BF03178334. - DOI
-
- Cocaign BM, Lindley ND. Pyruvate overflow and carbon flux within the central metabolic pathways of Corynebacterium glutamicum during growth on lactate. Amsterdam: Elsevier; 1995.
-
- de Graaf AA (2000) Metabolic flux analysis of Corynebacterium glutamicum. In: Schügerl KB, Bellgardt KH (ed) Bioreaction engineering, modelling and control. Springer, New york, pp 506–555
LinkOut - more resources
Full Text Sources
Other Literature Sources

