Frataxin-bypassing Isu1: characterization of the bypass activity in cells and mitochondria
- PMID: 24433162
- PMCID: PMC4021491
- DOI: 10.1042/BJ20131273
Frataxin-bypassing Isu1: characterization of the bypass activity in cells and mitochondria
Abstract
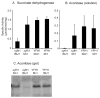
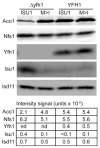
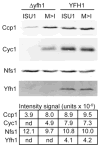
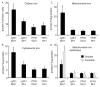
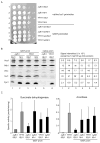
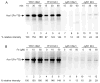
Frataxin is a conserved mitochondrial protein, and deficiency underlies the neurodegenerative disease Friedreich's ataxia. Frataxin interacts with the core machinery for Fe-S cluster assembly in mitochondria. Recently we reported that in frataxin-deleted yeast strains, a spontaneously occurring mutation in one of two genes encoding redundant Isu scaffold proteins, bypassed the mutant phenotypes. In the present study we created strains expressing a single scaffold protein, either Isu1 or the bypass mutant M107I Isu1. Our results show that in the frataxin-deletion strain expressing the bypass mutant Isu1, cell growth, Fe-S cluster protein activities, haem proteins and iron homoeostasis were restored to normal or close to normal. The bypass effects were not mediated by changes in Isu1 expression level. The persulfide-forming activity of the cysteine desulfurase was diminished in the frataxin deletion (∆yfh1 ISU1) and was improved by expression of the bypass Isu1 (∆yfh1 M107I ISU1). The addition of purified bypass M107I Isu1 protein to a ∆yfh1 lysate conferred similar enhancement of cysteine desulfurase as did frataxin, suggesting that this effect contributed to the bypass mechanism. Fe-S cluster-forming activity in isolated mitochondria was stimulated by the bypass Isu1, albeit at a lower rate. The rescuing effects of the bypass Isu1 point to ways that the core defects in Friedreich's ataxia mitochondria can be restored.
Figures



Similar articles
-
Mutation in the Fe-S scaffold protein Isu bypasses frataxin deletion.Biochem J. 2012 Jan 1;441(1):473-80. doi: 10.1042/BJ20111637. Biochem J. 2012. PMID: 21936771 Free PMC article.
-
Turning Saccharomyces cerevisiae into a Frataxin-Independent Organism.PLoS Genet. 2015 May 21;11(5):e1005135. doi: 10.1371/journal.pgen.1005135. eCollection 2015 May. PLoS Genet. 2015. PMID: 25996596 Free PMC article.
-
Frataxin directly stimulates mitochondrial cysteine desulfurase by exposing substrate-binding sites, and a mutant Fe-S cluster scaffold protein with frataxin-bypassing ability acts similarly.J Biol Chem. 2013 Dec 27;288(52):36773-86. doi: 10.1074/jbc.M113.525857. Epub 2013 Nov 11. J Biol Chem. 2013. PMID: 24217246 Free PMC article.
-
Frataxin and mitochondrial FeS cluster biogenesis.J Biol Chem. 2010 Aug 27;285(35):26737-26743. doi: 10.1074/jbc.R110.118679. Epub 2010 Jun 3. J Biol Chem. 2010. PMID: 20522547 Free PMC article. Review.
-
The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism.Biochim Biophys Acta. 2012 Sep;1823(9):1491-508. doi: 10.1016/j.bbamcr.2012.05.009. Epub 2012 May 15. Biochim Biophys Acta. 2012. PMID: 22609301 Review.
Cited by
-
Turning Escherichia coli into a Frataxin-Dependent Organism.PLoS Genet. 2015 May 21;11(5):e1005134. doi: 10.1371/journal.pgen.1005134. eCollection 2015 May. PLoS Genet. 2015. PMID: 25996492 Free PMC article.
-
Interactions of iron-bound frataxin with ISCU and ferredoxin on the cysteine desulfurase complex leading to Fe-S cluster assembly.J Inorg Biochem. 2018 Jun;183:107-116. doi: 10.1016/j.jinorgbio.2018.03.007. Epub 2018 Mar 15. J Inorg Biochem. 2018. PMID: 29576242 Free PMC article.
-
Mammalian iron-sulphur proteins: novel insights into biogenesis and function.Nat Rev Mol Cell Biol. 2015 Jan;16(1):45-55. doi: 10.1038/nrm3909. Epub 2014 Nov 26. Nat Rev Mol Cell Biol. 2015. PMID: 25425402 Review.
-
Mammalian Fe-S proteins: definition of a consensus motif recognized by the co-chaperone HSC20.Metallomics. 2016 Oct 1;8(10):1032-1046. doi: 10.1039/c6mt00167j. Metallomics. 2016. PMID: 27714045 Free PMC article. Review.
-
Fe-S cluster biogenesis in isolated mammalian mitochondria: coordinated use of persulfide sulfur and iron and requirements for GTP, NADH, and ATP.J Biol Chem. 2015 Jan 2;290(1):640-57. doi: 10.1074/jbc.M114.610402. Epub 2014 Nov 14. J Biol Chem. 2015. PMID: 25398879 Free PMC article.
References
-
- Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, et al. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–1427. - PubMed
-
- Wilson RB, Roof DM. Respiratory deficiency due to loss of mitochondrial DNA in yeast lacking the frataxin homologue. Nat Genet. 1997;16:352–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

