ACPA-positive and ACPA-negative rheumatoid arthritis differ in their requirements for combination DMARDs and corticosteroids: secondary analysis of a randomized controlled trial
- PMID: 24433430
- PMCID: PMC3979097
- DOI: 10.1186/ar4439
ACPA-positive and ACPA-negative rheumatoid arthritis differ in their requirements for combination DMARDs and corticosteroids: secondary analysis of a randomized controlled trial
Abstract
Introduction: UK guidelines recommend that all early active rheumatoid arthritis (RA) patients are offered combination disease-modifying antirheumatic drugs (DMARDs) and short-term corticosteroids. Anti-citrullinated protein antibody (ACPA)-positive and ACPA-negative RA may differ in their treatment responses. We used data from a randomized controlled trial - the Combination Anti-Rheumatic Drugs in Early RA (CARDERA) trial - to examine whether responses to intensive combination treatments in early RA differ by ACPA status.
Methods: The CARDERA trial randomized 467 early active RA patients to receive: (1) methotrexate, (2) methotrexate/ciclosporin, (3) methotrexate/prednisolone or (4) methotrexate/ciclosporin/prednisolone in a factorial-design. Patients were assessed every six months for two years. In this analysis we evaluated 431 patients with available ACPA status. To minimize multiple testing we used a mixed-effects repeated measures ANOVA model to test for an interaction between ACPA and treatment on mean changes from baseline for each outcome (Larsen, disease activity scores on a 28-joint count (DAS28), Health Assessment Questionnaire (HAQ), EuroQol, SF-36 physical component summary (PCS) and mental component summary (MCS) scores). When a significant interaction was present, mean changes in outcomes were compared by treatment group at each time point using t-tests stratified by ACPA status. Odds ratios (ORs) for the onset of new erosions with treatment were calculated stratified by ACPA.
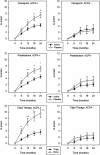
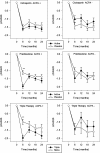
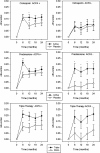
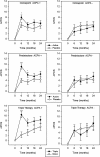
Results: ACPA status influenced the need for combination treatments to reduce radiological progression. ACPA-positive patients had significant reductions in Larsen score progression with all treatments. ACPA-positive patients receiving triple therapy had the greatest benefits: two-year mean Larsen score increases comprised 3.66 (95% confidence interval (CI) 2.27 to 5.05) with triple therapy and 9.58 (95% CI 6.76 to 12.39) with monotherapy; OR for new erosions with triple therapy versus monotherapy was 0.32 (95% CI 0.14 to 0.72; P = 0.003). ACPA-negative patients had minimal radiological progression irrespective of treatment. Corticosteroid's impact on improving DAS28/PCS scores was confined to ACPA-positive RA.
Conclusions: ACPA status influences the need for combination DMARDs and high-dose tapering corticosteroids in early RA. In CARDERA, combination therapy was only required to prevent radiological progression in ACPA-positive patients; corticosteroids only provided significant disease activity and physical health improvements in ACPA-positive disease. This suggests ACPA is an important biomarker for guiding treatment decisions in early RA.
Trial registration: Current Controlled Trials ISRCTN32484878.
Figures

References
-
- Eyre S, Bowes J, Diogo D, Lee A, Barton A, Martin P, Zhernakova A, Stahl E, Viatte S, McAllister K, Amos CI, Padyukov L, Toes RE, Huizinga TW, Wijmenga C, Trynka G, Franke L, Westra HJ, Alfredsson L, Hu X, Sandor C, de Bakker PI, Davila S, Khor CC, Heng KK, Andrews R, Edkins S, Hunt SE, Langford C, Symmons D. et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet. 2012;16:1336–1340. doi: 10.1038/ng.2462. - DOI - PMC - PubMed
-
- Padyukov L, Seielstad M, Ong RT, Ding B, Ronnelid J, Seddighzadeh M, Alfredsson L, Klareskog L. Epidemiological Investigation of Rheumatoid Arthritis study group: a genome-wide association study suggests contrasting associations in ACPA-positive versus ACPA-negative rheumatoid arthritis. Ann Rheum Dis. 2011;16:259–265. doi: 10.1136/ard.2009.126821. - DOI - PMC - PubMed
-
- Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, Ronnelid J, Harris HE, Ulfgren A-K, Rantapaa-Dahlqvist S, Eklund A, Padyukov L, Alfredsson L. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;16:38–46. doi: 10.1002/art.21575. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

