Mechanisms underlying the neuronal-based symptoms of allergy
- PMID: 24433703
- PMCID: PMC6483074
- DOI: 10.1016/j.jaci.2013.11.027
Mechanisms underlying the neuronal-based symptoms of allergy
Abstract
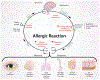
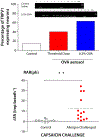
Persons with allergies present with symptoms that often are the result of alterations in the nervous system. Neuronally based symptoms depend on the organ in which the allergic reaction occurs but can include red itchy eyes, sneezing, nasal congestion, rhinorrhea, coughing, bronchoconstriction, airway mucus secretion, dysphagia, altered gastrointestinal motility, and itchy swollen skin. These symptoms occur because mediators released during an allergic reaction can interact with sensory nerves, change processing in the central nervous system, and alter transmission in sympathetic, parasympathetic, and enteric autonomic nerves. In addition, evidence supports the idea that in some subjects this neuromodulation is, for reasons poorly understood, upregulated such that the same degree of nerve stimulus causes a larger effect than seen in healthy subjects. There are distinctions in the mechanisms and nerve types involved in allergen-induced neuromodulation among different organ systems, but general principles have emerged. The products of activated mast cells, other inflammatory cells, and resident cells can overtly stimulate nerve endings, cause long-lasting changes in neuronal excitability, increase synaptic efficacy, and also change gene expression in nerves, resulting in phenotypically altered neurons. A better understanding of these processes might lead to novel therapeutic strategies aimed at limiting the suffering of those with allergies.
Keywords: Allergy; C-fiber; IgE; action potential; afferent; airways; allergen; anti-inflammatory; autonomic; bronchospasm; cough; critical periods; cytokine; degranulation; depolarization; dorsal root; dysfunction; efferent; enteric; eye; ganglia; gut; histamine; hypersecretion; inflammation; innervation; ion channel; irritant; itch; jugular; leukotriene; lungs; mast cell; mast cell mediators; mast cell-nerve interactions; motility; myenteric; nerve; nociceptor; nodose; nucleus tractus solitarious; pain; parasympathetic; phenotypic switch; plasticity; prostaglandin; receptor; reflex; sensory; skin; steroids; sympathetic; symptoms; transient receptor potential channels; trigeminal; vagal.
Copyright © 2014 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: B. Undem has received grants from the National Institutes of Health and GlaxoSmithKline; is on the scientific advisory board for Afferent Pharmaceuticals; and has consultant arrangements with GlaxoSmithKline. T. Taylor-Clark declares that he has no relevant conflicts of interest.
Figures






References
-
- Lynn B Somatosensory receptors and their CNS connections. Annu Rev Physiol 1975;37:105–27. - PubMed
-
- Sherrington C The integrative action of the nervous system. New Haven (CT): Yale University Press; 1906.
-
- Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 1984; 99:1–110. - PubMed
-
- Wood JD. Enteric neuroimmunophysiology and pathophysiology. Gastroenterology 2004;127:635–57. - PubMed
-
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil 2007;19:1–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

