Myocardial contraction and hyaluronic acid mechanotransduction in epithelial-to-mesenchymal transformation of endocardial cells
- PMID: 24433835
- PMCID: PMC3950274
- DOI: 10.1016/j.biomaterials.2013.12.051
Myocardial contraction and hyaluronic acid mechanotransduction in epithelial-to-mesenchymal transformation of endocardial cells
Abstract
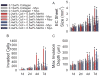
Epithelial-to-mesenchymal transition (EMT) of endocardial cells is a critical initial step in the formation of heart valves. The collagen gel in vitro model has provided significant information on the role of growth factors regulating EMT but has not permitted investigation of mechanical factors. Therefore we sought to develop a system to probe the effects of mechanical inputs on endocardial EMT by incorporating hyaluronic acid (HA), the primary component of endocardial cushions in developing heart valves, into the gel assay. This was achieved using a combination collagen and crosslinkable methacrylated HA hydrogel (Coll-MeHA). Avian atrioventricular canal explants on Coll-MeHA gels showed increased numbers of transformed cells. Analysis of the mechanical properties of Coll-MeHA gels shows that stiffness does not directly affect EMT. Hydrogel deformation from the beating myocardium of explants directly led to higher levels of regional gel deformation and larger average strain magnitudes associated with invaded cells on Coll-MeHA gels. Inhibition of this contraction reduced EMT on all gel types, although to a lesser extent on Coll-MeHA gels. Using the system we have developed, which permits the manipulation of mechanical factors, we have demonstrated that active mechanical forces play a role in the regulation of endocardial EMT.
Keywords: Collagen; Epithelial-to-mesenchymal transition (EMT); Heart valve; Hyaluronic acid; Mechanical properties.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
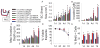



Similar articles
-
Functional BMP receptor in endocardial cells is required in atrioventricular cushion mesenchymal cell formation in chick.Dev Biol. 2007 Jun 1;306(1):179-92. doi: 10.1016/j.ydbio.2007.03.015. Epub 2007 Mar 16. Dev Biol. 2007. PMID: 17449024 Free PMC article.
-
Analysis of the endocardial-to-mesenchymal transformation of heart valve development by collagen gel culture assay.Methods Mol Biol. 2012;843:101-9. doi: 10.1007/978-1-61779-523-7_10. Methods Mol Biol. 2012. PMID: 22222525
-
3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity.PLoS One. 2017 Jun 6;12(6):e0177628. doi: 10.1371/journal.pone.0177628. eCollection 2017. PLoS One. 2017. PMID: 28586346 Free PMC article.
-
Collagen gel analysis of epithelial-mesenchymal transition in the embryo heart: an in vitro model system for the analysis of tissue interaction, signal transduction, and environmental effects.Birth Defects Res C Embryo Today. 2011 Dec;93(4):298-311. doi: 10.1002/bdrc.20222. Birth Defects Res C Embryo Today. 2011. PMID: 22271679 Review.
-
Nfatc1 directs the endocardial progenitor cells to make heart valve primordium.Trends Cardiovasc Med. 2013 Nov;23(8):294-300. doi: 10.1016/j.tcm.2013.04.003. Epub 2013 May 10. Trends Cardiovasc Med. 2013. PMID: 23669445 Free PMC article. Review.
Cited by
-
Cancer-associated fibroblasts support vascular growth through mechanical force.Sci Rep. 2017 Oct 3;7(1):12574. doi: 10.1038/s41598-017-13006-x. Sci Rep. 2017. PMID: 28974764 Free PMC article.
-
Biomechanical stimulation promotes blood vessel growth despite VEGFR-2 inhibition.BMC Biol. 2023 Dec 10;21(1):290. doi: 10.1186/s12915-023-01792-y. BMC Biol. 2023. PMID: 38072992 Free PMC article.
-
Hyaluronic acid regulates heart valve interstitial cell contraction in fibrin-based scaffolds.Acta Biomater. 2021 Dec;136:124-136. doi: 10.1016/j.actbio.2021.09.046. Epub 2021 Sep 28. Acta Biomater. 2021. PMID: 34592445 Free PMC article.
-
Rhythms of growth: unveiling the mechanobiology behind heart maturation.J Physiol. 2025 Apr 11:10.1113/JP287905. doi: 10.1113/JP287905. Online ahead of print. J Physiol. 2025. PMID: 40215090 Review.
-
Cardiac valve cells and their microenvironment--insights from in vitro studies.Nat Rev Cardiol. 2014 Dec;11(12):715-27. doi: 10.1038/nrcardio.2014.162. Epub 2014 Oct 14. Nat Rev Cardiol. 2014. PMID: 25311230 Free PMC article. Review.
References
-
- Barnett JV, Desgrosellier JS. Early events in valvulogenesis: A signaling perspective. Birth Defects Res C Embryo Today. 2003;69(1):58–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

