Cytolethal distending toxin B as a cell-killing component of tumor-targeted anthrax toxin fusion proteins
- PMID: 24434511
- PMCID: PMC4040664
- DOI: 10.1038/cddis.2013.540
Cytolethal distending toxin B as a cell-killing component of tumor-targeted anthrax toxin fusion proteins
Abstract
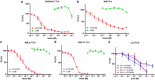
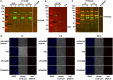
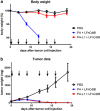
Cytolethal distending toxin (Cdt) is produced by Gram-negative bacteria of several species. It is composed of three subunits, CdtA, CdtB, and CdtC, with CdtB being the catalytic subunit. We fused CdtB from Haemophilus ducreyi to the N-terminal 255 amino acids of Bacillus anthracis toxin lethal factor (LFn) to design a novel, potentially potent antitumor drug. As a result of this fusion, CdtB was transported into the cytosol of targeted cells via the efficient delivery mechanism of anthrax toxin. The fusion protein efficiently killed various human tumor cell lines by first inducing a complete cell cycle arrest in the G2/M phase, followed by induction of apoptosis. The fusion protein showed very low toxicity in mouse experiments and impressive antitumor effects in a Lewis Lung carcinoma model, with a 90% cure rate. This study demonstrates that efficient drug delivery by a modified anthrax toxin system combined with the enzymatic activity of CdtB has great potential as anticancer treatment and should be considered for the development of novel anticancer drugs.
Figures


Similar articles
-
CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity.Infect Immun. 2001 Jul;69(7):4358-65. doi: 10.1128/IAI.69.7.4358-4365.2001. Infect Immun. 2001. PMID: 11401974 Free PMC article.
-
A CdtA-CdtC complex can block killing of HeLa cells by Haemophilus ducreyi cytolethal distending toxin.Infect Immun. 2003 Nov;71(11):6633-40. doi: 10.1128/IAI.71.11.6633-6640.2003. Infect Immun. 2003. PMID: 14573688 Free PMC article.
-
Haemophilus parasuis cytolethal distending toxin induces cell cycle arrest and p53-dependent apoptosis.PLoS One. 2017 May 18;12(5):e0177199. doi: 10.1371/journal.pone.0177199. eCollection 2017. PLoS One. 2017. PMID: 28545143 Free PMC article.
-
Molecular Mechanisms and Potential Clinical Applications of Campylobacter jejuni Cytolethal Distending Toxin.Front Cell Infect Microbiol. 2016 Feb 9;6:9. doi: 10.3389/fcimb.2016.00009. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 26904508 Free PMC article. Review.
-
The contribution of cytolethal distending toxin to bacterial pathogenesis.Crit Rev Microbiol. 2006 Oct-Dec;32(4):227-48. doi: 10.1080/10408410601023557. Crit Rev Microbiol. 2006. PMID: 17123907 Review.
Cited by
-
Selective targeting of metastatic ovarian cancer using an engineered anthrax prodrug activated by membrane-anchored serine proteases.Proc Natl Acad Sci U S A. 2022 Jul 12;119(28):e2201423119. doi: 10.1073/pnas.2201423119. Epub 2022 Jul 8. Proc Natl Acad Sci U S A. 2022. PMID: 35867758 Free PMC article.
-
Engineering of Cytolethal Distending Toxin B by Its Reducing Immunogenicity and Maintaining Stability as a New Drug Candidate for Tumor Therapy; an In Silico Study.Toxins (Basel). 2021 Nov 5;13(11):785. doi: 10.3390/toxins13110785. Toxins (Basel). 2021. PMID: 34822569 Free PMC article.
-
Prevalence estimates of Helicobacter species infection in pancreatic and biliary tract cancers.Helicobacter. 2022 Feb;27(1):e12866. doi: 10.1111/hel.12866. Epub 2022 Jan 10. Helicobacter. 2022. PMID: 35005807 Free PMC article.
-
Targeting the membrane-anchored serine protease testisin with a novel engineered anthrax toxin prodrug to kill tumor cells and reduce tumor burden.Oncotarget. 2015 Oct 20;6(32):33534-53. doi: 10.18632/oncotarget.5214. Oncotarget. 2015. PMID: 26392335 Free PMC article.
-
Augmenting the Efficacy of Immunotoxins and Other Targeted Protein Toxins by Endosomal Escape Enhancers.Toxins (Basel). 2016 Jul 1;8(7):200. doi: 10.3390/toxins8070200. Toxins (Basel). 2016. PMID: 27376327 Free PMC article. Review.
References
-
- Wising C, Molne L, Jonsson IM, Ahlman K, Lagergard T. The cytolethal distending toxin of Haemophilus ducreyi aggravates dermal lesions in a rabbit model of chancroid. Microbes Infection/Institut Pasteur. 2005;7:867–874. - PubMed
-
- Lagergard T, Lundqvist A, Wising C, Gabrielsson V, Ahlman K. Formaldehyde treatment increases the immunogenicity and decreases the toxicity of Haemophilus ducreyi cytolethal distending toxin. Vaccine. 2007;25:3606–3614. - PubMed
-
- Anderson JD, MacNab AJ, Gransden WR, Damm SM, Johnson WM, Lior H. Gastroenteritis and encephalopathy associated with a strain of Escherichia coli 055:K59:H4 that produced a cytolethal distending toxin. Pediatr Infect Dis J. 1987;6:1135–1136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

