Depletion of RIPK3 or MLKL blocks TNF-driven necroptosis and switches towards a delayed RIPK1 kinase-dependent apoptosis
- PMID: 24434512
- PMCID: PMC4040672
- DOI: 10.1038/cddis.2013.531
Depletion of RIPK3 or MLKL blocks TNF-driven necroptosis and switches towards a delayed RIPK1 kinase-dependent apoptosis
Abstract
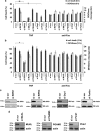
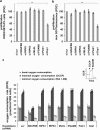
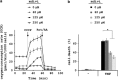
In human cells, the RIPK1-RIPK3-MLKL-PGAM5-Drp1 axis drives tumor necrosis factor (TNF)-induced necroptosis through mitochondrial fission, but whether this pathway is conserved among mammals is not known. To answer this question, we analyzed the presence and functionality of the reported necroptotic axis in mice. As in humans, knockdown of receptor-interacting kinase-3 (RIPK3) or mixed lineage kinase domain like (MLKL) blocks TNF-induced necroptosis in L929 fibrosarcoma cells. However, repression of either of these proteins did not protect the cells from death, but instead induced a switch from TNF-induced necroptosis to receptor-interacting kinase-1 (RIPK1) kinase-dependent apoptosis. In addition, although mitochondrial fission also occurs during TNF-induced necroptosis in L929 cells, we found that knockdown of phosphoglycerate mutase 5 (PGAM5) and dynamin 1 like protein (Drp1) did not markedly protect the cells from TNF-induced necroptosis. Depletion of Pink1, a reported interactor of both PGAM5 and Drp1, did not affect TNF-induced necroptosis. These results indicate that in these murine cells mitochondrial fission and Pink1 dependent processes, including Pink-Parkin dependent mitophagy, apparently do not promote necroptosis. Our data demonstrate that the core components of the necrosome (RIPK1, RIPK3 and MLKL) are crucial to induce TNF-dependent necroptosis both in human and in mouse cells, but the associated mechanisms may differ between the two species or cell types.
Figures

Similar articles
-
Necroptosis induced by RIPK3 requires MLKL but not Drp1.Cell Death Dis. 2014 Feb 27;5(2):e1086. doi: 10.1038/cddis.2014.18. Cell Death Dis. 2014. PMID: 24577084 Free PMC article.
-
The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism.Immunity. 2013 Sep 19;39(3):443-53. doi: 10.1016/j.immuni.2013.06.018. Epub 2013 Sep 5. Immunity. 2013. PMID: 24012422
-
FKBP12 mediates necroptosis by initiating RIPK1-RIPK3-MLKL signal transduction in response to TNF receptor 1 ligation.J Cell Sci. 2019 May 20;132(10):jcs227777. doi: 10.1242/jcs.227777. J Cell Sci. 2019. PMID: 31028177
-
Viral-induced neuronal necroptosis: Detrimental to brain function and regulation by necroptosis inhibitors.Biochem Pharmacol. 2023 Jul;213:115591. doi: 10.1016/j.bcp.2023.115591. Epub 2023 May 16. Biochem Pharmacol. 2023. PMID: 37196683 Review.
-
The Inflammatory Signal Adaptor RIPK3: Functions Beyond Necroptosis.Int Rev Cell Mol Biol. 2017;328:253-275. doi: 10.1016/bs.ircmb.2016.08.007. Epub 2016 Sep 22. Int Rev Cell Mol Biol. 2017. PMID: 28069136 Free PMC article. Review.
Cited by
-
An outline of necrosome triggers.Cell Mol Life Sci. 2016 Jun;73(11-12):2137-52. doi: 10.1007/s00018-016-2189-y. Epub 2016 Apr 6. Cell Mol Life Sci. 2016. PMID: 27052312 Free PMC article. Review.
-
Regulation of RIPK3- and RHIM-dependent Necroptosis by the Proteasome.J Biol Chem. 2016 Mar 11;291(11):5948-5959. doi: 10.1074/jbc.M115.700997. Epub 2016 Jan 19. J Biol Chem. 2016. PMID: 26786097 Free PMC article.
-
Phosphorylation of caspase-8 by RSKs via organ-constrained effects controls the sensitivity to TNF-induced death.Cell Death Discov. 2024 May 24;10(1):255. doi: 10.1038/s41420-024-02024-0. Cell Death Discov. 2024. PMID: 38789425 Free PMC article.
-
Necroptosis in development, inflammation and disease.Nat Rev Mol Cell Biol. 2017 Feb;18(2):127-136. doi: 10.1038/nrm.2016.149. Epub 2016 Dec 21. Nat Rev Mol Cell Biol. 2017. PMID: 27999438 Review.
-
Absence of RIPK3 predicts necroptosis resistance in malignant melanoma.Cell Death Dis. 2015 Sep 10;6(9):e1884. doi: 10.1038/cddis.2015.240. Cell Death Dis. 2015. PMID: 26355347 Free PMC article.
References
-
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. - PubMed
-
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

