Prostasin may contribute to chemoresistance, repress cancer cells in ovarian cancer, and is involved in the signaling pathways of CASP/PAK2-p34/actin
- PMID: 24434518
- PMCID: PMC4043260
- DOI: 10.1038/cddis.2013.523
Prostasin may contribute to chemoresistance, repress cancer cells in ovarian cancer, and is involved in the signaling pathways of CASP/PAK2-p34/actin
Abstract
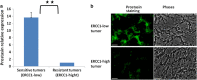
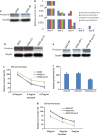
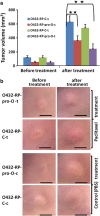
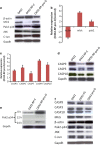
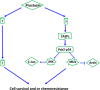
Ovarian cancer is the deadliest of gynecologic cancers, largely due to the development of drug resistance in chemotherapy. Prostasin may have an essential role in the oncogenesis. In this study, we show that prostasin is decreased in an ovarian cancer drug-resistant cell line and in ovarian cancer patients with high levels of excision repair cross-complementing 1, a marker for chemoresistance. Our cell cultural model investigation demonstrates prostasin has important roles in the development of drug resistance and cancer cell survival. Forced overexpression of prostasin in ovarian cancer cells greatly induces cell death (resulting in 99% cell death in a drug-resistant cell line and 100% cell death in other tested cell lines). In addition, the surviving cells grow at a much lower rate compared with non-overexpressed cells. In vivo studies indicate that forced overexpression of prostasin in drug-resistant cells greatly inhibits the growth of tumors and may partially reverse drug resistance. Our investigation of the molecular mechanisms suggests that prostasin may repress cancer cells and/or contribute to chemoresistance by modulating the CASP/P21-activated protein kinase (PAK2)-p34 pathway, and thereafter PAK2-p34/JNK/c-jun and PAK2-p34/mlck/actin signaling pathways. Thus, we introduce prostain as a potential target for treating/repressing some ovarian tumors and have begun to identify their relevant molecular targets in specific signaling pathways.
Figures
Similar articles
-
The serine protease prostasin (PRSS8) is a potential biomarker for early detection of ovarian cancer.J Ovarian Res. 2016 Mar 31;9:20. doi: 10.1186/s13048-016-0228-9. J Ovarian Res. 2016. PMID: 27036110 Free PMC article.
-
PSP94, an upstream signaling mediator of prostasin found highly elevated in ovarian cancer.Cell Death Dis. 2014 Sep 4;5(9):e1407. doi: 10.1038/cddis.2014.374. Cell Death Dis. 2014. PMID: 25188517 Free PMC article.
-
Regulation of HtrA2/Omi by X-linked inhibitor of apoptosis protein in chemoresistance in human ovarian cancer cells.Gynecol Oncol. 2005 May;97(2):413-21. doi: 10.1016/j.ygyno.2004.12.055. Gynecol Oncol. 2005. PMID: 15863139
-
PAK2 as a therapeutic target in cancer: Mechanisms, challenges, and future perspectives.Biochim Biophys Acta Rev Cancer. 2025 Feb;1880(1):189246. doi: 10.1016/j.bbcan.2024.189246. Epub 2024 Dec 16. Biochim Biophys Acta Rev Cancer. 2025. PMID: 39694422 Review.
-
Mechanisms of Drug Resistance in Ovarian Cancer and Associated Gene Targets.Cancers (Basel). 2022 Dec 18;14(24):6246. doi: 10.3390/cancers14246246. Cancers (Basel). 2022. PMID: 36551731 Free PMC article. Review.
Cited by
-
Regulation of Cancer Metastasis by PAK2.Int J Mol Sci. 2024 Dec 15;25(24):13443. doi: 10.3390/ijms252413443. Int J Mol Sci. 2024. PMID: 39769207 Free PMC article. Review.
-
Hyperactivation of p21-Activated Kinases in Human Cancer and Therapeutic Sensitivity.Biomedicines. 2023 Feb 5;11(2):462. doi: 10.3390/biomedicines11020462. Biomedicines. 2023. PMID: 36830998 Free PMC article. Review.
-
Mechanisms of the JNK/p38 MAPK signaling pathway in drug resistance in ovarian cancer.Front Oncol. 2025 Apr 24;15:1533352. doi: 10.3389/fonc.2025.1533352. eCollection 2025. Front Oncol. 2025. PMID: 40352594 Free PMC article. Review.
-
A bead-based cleavage method for large-scale identification of protease substrates.Sci Rep. 2016 Mar 3;6:22645. doi: 10.1038/srep22645. Sci Rep. 2016. PMID: 26935269 Free PMC article.
-
P21 activated kinase 2 promotes pancreatic cancer growth and metastasis.Oncol Lett. 2019 Apr;17(4):3709-3718. doi: 10.3892/ol.2019.10040. Epub 2019 Feb 14. Oncol Lett. 2019. Retraction in: Oncol Lett. 2023 Jul 10;26(2):363. doi: 10.3892/ol.2023.13949. PMID: 30930982 Free PMC article. Retracted.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Thigpen T. First-line therapy for ovarian carcinoma: what's next. Cancer Invest. 2004;22 (Suppl 2:21–28. - PubMed
-
- Markman M, Liu PY, Wilczynski S, Monk B, Copeland LJ, Alvarez RD, et al. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. J Clin Oncol. 2003;21:2460–2465. - PubMed
-
- Mei L, Chen H, Wei DM, Fang F, Liu GJ, Xie HY, et al. Maintenance chemotherapy for ovarian cancer. Cochrane Database Syst Rev. 2010;9:CD007414. - PubMed
-
- Kikuchi Y, Kita T, Takano M, Kudoh K, Yamamoto K. Treatment options in the management of ovarian cancer. Expert Opin Pharmacother. 2005;6:743–754. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

