Depletion of myeloid-derived suppressor cells during interleukin-12 immunogene therapy does not confer a survival advantage in experimental malignant glioma
- PMID: 24434573
- PMCID: PMC4035218
- DOI: 10.1038/cgt.2013.81
Depletion of myeloid-derived suppressor cells during interleukin-12 immunogene therapy does not confer a survival advantage in experimental malignant glioma
Abstract
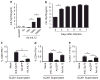
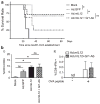
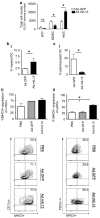
Myeloid-derived suppressor cells (MDSCs) accumulate in the glioma microenvironment during tumor progression and promote immunosuppression. Interleukin-12 (IL-12) immunogene therapy can alter MDSCs toward an antigen-presenting cell phenotype and these mature cells can have a central role in antigen presentation. It remains unclear, however, how MDSC depletion can affect glioma immunotherapy. In this study, we generated a replication-deficient adenoviral vector, Ad.5/3.cRGD-mIL12p70, that transduces the GL261-based murine glioma cell line, resulting in the induction of biologically active, murine IL12p70 expression. Ex vivo, IL-12 expressed by GL261 cells induced interferon-γ synthesis in CD8(+) T cells (P<0.001), CD4(+) T cells (P=0.009) and natural killer cells (P=0.036). When injected 1 week after tumor implantation, Ad.5/3.cRGD-mIL12p70 successfully prolonged the survival of glioma-bearing mice. Sixty percent of animals treated with IL-12 immunotherapy were long-term survivors over 175 days, whereas all the control group animals expired by 40 days after tumor implantation (P=0.026). Mice receiving Ad.5/3.cRGD-mIL12p70 also accumulated 50% less MDSCs in the brain than the control group (P=0.007). Moreover, in the IL-12 group, MDSCs significantly overexpressed CD80 and major histocompatibility complex class II molecules (P=0.041). Depletion of MDSCs with Gr1(+) antibody had no survival benefit induced by IL-12-mediated immunotherapy. Of note, IL-12 therapy increased the presence of myeloid dendritic cells (mDCs) in the glioma microenvironment (P=0.0069). Ultimately, the data show that in the context of IL-12 immunogene therapy, MDSCs are dispensable and mDCs may provide the majority of antigen presentation in the brain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Malmstrom A, Gronberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–926. - PubMed
-
- Becker KP, Yu J. Status quo—standard-of-care medical and radiation therapy for glioblastoma. Cancer J. 2012;18:12–19. - PubMed
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
-
- Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–4729. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

