A Pil1-Sle1-Syj1-Tax4 functional pathway links eisosomes with PI(4,5)P2 regulation
- PMID: 24434583
- PMCID: PMC3953819
- DOI: 10.1242/jcs.143545
A Pil1-Sle1-Syj1-Tax4 functional pathway links eisosomes with PI(4,5)P2 regulation
Abstract
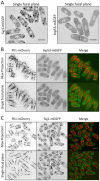
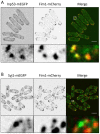
Stable compartments of the plasma membrane promote a wide range of cellular functions. In yeast cells, cytosolic structures called eisosomes generate prominent cortical invaginations of unknown function. Through a series of genetic screens in fission yeast, we found that the eisosome proteins Pil1 and Sle1 function with the synaptojanin-like lipid phosphatase Syj1 and its ligand Tax4. This genetic pathway connects eisosome function with the hydrolysis of phosphatidylinositol (4,5)-bisphosphate [PI(4,5)P2] in cells. Defects in PI(4,5)P2 regulation led to eisosome defects, and we found that the core eisosome protein Pil1 can bind to and tubulate liposomes containing PI(4,5)P2. Mutations in components of the Pil1-Sle1-Syj1-Tax4 pathway suppress the growth and morphology defects of TORC2 mutants, indicating that eisosome-dependent regulation of PI(4,5)P2 feeds into signal transduction pathways. We propose that the geometry of membrane invaginations generates spatial and temporal signals for lipid-mediated signaling events in cells.
Keywords: Eisosome; PI(4,5)P2; Synaptojanin; TORC2.
Figures


References
-
- Aguilar P. S., Fröhlich F., Rehman M., Shales M., Ulitsky I., Olivera-Couto A., Braberg H., Shamir R., Walter P., Mann M. et al. (2010). A plasma-membrane E-MAP reveals links of the eisosome with sphingolipid metabolism and endosomal trafficking. Nat. Struct. Mol. Biol. 17, 901–908 10.1038/nsmb.1829 - DOI - PMC - PubMed
-
- Bähler J., Wu J. Q., Longtine M. S., Shah N. G., McKenzie A., 3rd, Steever A. B., Wach A., Philippsen P., Pringle J. R. (1998). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943–951 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

