Bacterial collagen-like proteins that form triple-helical structures
- PMID: 24434612
- PMCID: PMC4096566
- DOI: 10.1016/j.jsb.2014.01.003
Bacterial collagen-like proteins that form triple-helical structures
Abstract
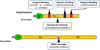
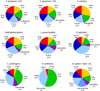
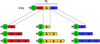
A large number of collagen-like proteins have been identified in bacteria during the past 10years, principally from analysis of genome databases. These bacterial collagens share the distinctive Gly-Xaa-Yaa repeating amino acid sequence of animal collagens which underlies their unique triple-helical structure. A number of the bacterial collagens have been expressed in Escherichia coli, and they all adopt a triple-helix conformation. Unlike animal collagens, these bacterial proteins do not contain the post-translationally modified amino acid, hydroxyproline, which is known to stabilize the triple-helix structure and may promote self-assembly. Despite the absence of collagen hydroxylation, the triple-helix structures of the bacterial collagens studied exhibit a high thermal stability of 35-39°C, close to that seen for mammalian collagens. These bacterial collagens are readily produced in large quantities by recombinant methods, either in the original amino acid sequence or in genetically manipulated sequences. This new family of recombinant, easy to modify collagens could provide a novel system for investigating structural and functional motifs in animal collagens and could also form the basis of new biomedical materials with designed structural properties and functions.
Keywords: Biomedical material; Collagen; Prokaryote; Recombinant expression; Thermal stability; Triple-helix.
Copyright © 2014. Published by Elsevier Inc.
Figures
References
-
- Adachi E, Katsumato O, Yamashina S, Prockop DJ, Fertala A. Collagen II containing a Cys substitution for Arg-alpha1-519. Analysis by atomic force microscopy demonstrates that mutated monomers alter the topography of the surface of collagen II fibrils. Matrix Biol. 1999;18:189–196. - PubMed
-
- Arnold WV, Fertala A, Sieron AL, Hattori H, Mechling D, Bächinger HP, Prockop DJ. Recombinant procollagen II: Deletion of D period segments identifies sequences that are required for helix stabilization and generates a temperature-sensitive N-proteinase cleavage site. J. Biol. Chem. 1998;273:31822–31828. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

