Liver-specific agents for contrast-enhanced MRI: role in oncological imaging
- PMID: 24434892
- PMCID: PMC3893895
- DOI: 10.1102/1470-7330.2013.0050
Liver-specific agents for contrast-enhanced MRI: role in oncological imaging
Abstract
Liver-specific magnetic resonance (MR) contrast agents are increasingly used in evaluation of the liver. They are effective in detection and morphological characterization of lesions, and can be useful for evaluation of biliary tree anatomy and liver function. The typical appearances and imaging pitfalls of various tumours at MR imaging performed with these agents can be understood by the interplay of pharmacokinetics of these contrast agents and transporter expression of the tumour. This review focuses on the applications of these agents in oncological imaging.
Figures


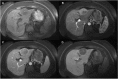


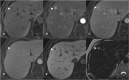
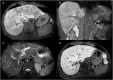
References
-
- Oudkerk M, Torres CG, Song B, et al. Characterization of liver lesions with mangafodipir trisodium-enhanced MR imaging: multicenter study comparing MR and dual-phase spiral CT. Radiology. 2002;223:517–524. - PubMed
-
- Koh KC, Kim HJ, Choe WH, et al. The clinical usefulness of SPIO-MRI in detection and staging of hepatocellular carcinoma. Taehan Kan Hakhoe Chi. 2003;9:17–24. - PubMed
-
- Reimer P, Schneider G, Schima W. Hepatobiliary contrast agents for contrast-enhanced MRI of the liver: properties, clinical development and applications. Eur Radiol. 2004;14:559–578. - PubMed
-
- Gandhi SN, Brown MA, Wong JG, Aguirre DA, Sirlin CB. MR contrast agents for liver imaging: what, when, how. Radiographics. 2006;26:1621–1636. - PubMed
-
- Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann H-J. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
