Type I interferon plays opposing roles in cytotoxicity and interferon-γ production by natural killer and CD8 T cells after influenza A virus infection in mice
- PMID: 24435166
- PMCID: PMC6741478
- DOI: 10.1159/000356824
Type I interferon plays opposing roles in cytotoxicity and interferon-γ production by natural killer and CD8 T cells after influenza A virus infection in mice
Abstract
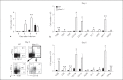
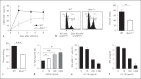
Type I interferons (IFNs) promote natural killer (NK) and CD8(+) T-cell responses, which play a role not only in the resolution of infection but also in the induction of acute lung injury following influenza A virus infection. We show here that IFN-α receptor knock-out (Ifnar1(-/-)) mice exhibited impaired cytotoxic activity as well as an increased ability of NK and CD8(+) T cells to produce IFN-γ after infection with influenza virus A/FM/1/47 (H1N1, a mouse-adapted strain). A deficiency in IFNAR signaling significantly impaired IL-10 production in influenza virus-infected lungs and enhanced IFN-γ production by NK cells, which were suppressed by exogenous IL-10. Depletion of NK cells but not CD8(+) T cells in Ifnar1(-/-) mice improved the survival rate after A/FM/1/47 infection, indicating that NK cells are responsible for acute lung injury in Ifnar1(-/-) mice following influenza A virus infection, although the depletion of IFN-γ did not improve the outcome. Thus, type I IFN signaling plays a role not only in the upregulation of cytotoxicity but also in the downregulation of some effector mechanisms including IFN-γ production by NK and CD8(+) T cells via IL-10 production.
© 2014 S. Karger AG, Basel.
Figures



References
-
- Abdul-Careem MF, Mian MF, Yue G, Gillgrass A, Chenoweth MJ, Barra NG, Chew MV, Chan T, Al-Garawi AA, Jordana M, Ashkar AA. Critical role of natural killer cells in lung immunopathology during influenza infection in mice. J Infect Dis. 2012;206:167–177. - PubMed
-
- Lettau M, Schmidt H, Kabelitz D, Janssen O. Secretory lysosomes and their cargo in T and NK cells. Immunol Lett. 2007;108:10–19. - PubMed
-
- Billiau A, Matthys P. Interferon-gamma: a historical perspective. Cytokine Growth Factor Rev. 2009;20:97–113. - PubMed
-
- Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

