Application of physiologically based absorption modeling to formulation development of a low solubility, low permeability weak base: mechanistic investigation of food effect
- PMID: 24435225
- PMCID: PMC3969476
- DOI: 10.1208/s12249-014-0075-1
Application of physiologically based absorption modeling to formulation development of a low solubility, low permeability weak base: mechanistic investigation of food effect
Abstract
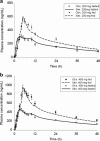
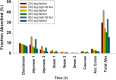
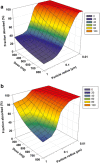
Physiologically based pharmacokinetic (PBPK) modeling has been broadly used to facilitate drug development, hereby we developed a PBPK model to systematically investigate the underlying mechanisms of the observed positive food effect of compound X (cpd X) and to strategically explore the feasible approaches to mitigate the food effect. Cpd X is a weak base with pH-dependent solubility; the compound displays significant and dose-dependent food effect in humans, leading to a nonadherence of drug administration. A GastroPlus Opt logD Model was selected for pharmacokinetic simulation under both fasted and fed conditions, where the biopharmaceutic parameters (e.g., solubility and permeability) for cpd X were determined in vitro, and human pharmacokinetic disposition properties were predicted from preclinical data and then optimized with clinical pharmacokinetic data. A parameter sensitivity analysis was performed to evaluate the effect of particle size on the cpd X absorption. A PBPK model was successfully developed for cpd X; its pharmacokinetic parameters (e.g., C max, AUCinf, and t max) predicted at different oral doses were within ±25% of the observed mean values. The in vivo solubility (in duodenum) and mean precipitation time under fed conditions were estimated to be 7.4- and 3.4-fold higher than those under fasted conditions, respectively. The PBPK modeling analysis provided a reasonable explanation for the underlying mechanism for the observed positive food effect of the cpd X in humans. Oral absorption of the cpd X can be increased by reducing the particle size (<100 nm) of an active pharmaceutical ingredient under fasted conditions and therefore, reduce the cpd X food effect correspondingly.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

