Long term duration of protective effect for HPV negative women: follow-up of primary HPV screening randomised controlled trial
- PMID: 24435414
- PMCID: PMC3898575
- DOI: 10.1136/bmj.g130
Long term duration of protective effect for HPV negative women: follow-up of primary HPV screening randomised controlled trial
Abstract
Objectives: To assess whether the increased sensitivity of screening for human papillomavirus (HPV) may represent overdiagnosis and to compare the long term duration of protective effect against cervical intraepithelial neoplasia grade 2 or worse (CIN2+) in HPV based and cytology based screening.
Design: 13 year follow-up of the Swedescreen randomised controlled trial of primary HPV screening.
Setting: Organised cervical screening programme in Sweden.
Participants: 12,527 women aged 32-38 attending organised screening were enrolled and randomised to HPV and cytology double testing (intervention arm, n=6257) or to cytology only, with samples frozen for future HPV testing (control arm, n=6270).
Main outcome measures: Cumulative incidence of CIN2+ and CIN3+ (Kaplan Meier curves). Longitudinal test characteristics were calculated for cytology only, HPV testing only, and cytology and HPV testing combined, adjusting for censoring.
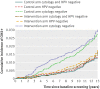
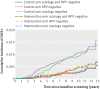
Results: The increased detection of CIN2+ in the intervention arm decreased over time. After six years, the cumulative incidence of CIN3+ was similar in both trial arms, and after 11 years the cumulative incidence of CIN2+ became similar in both arms. The longitudinal sensitivity of cytology for CIN2+ in the control arm at three years was similar to the sensitivity of HPV testing in the intervention arm at five years of follow-up: 85.94% (95% confidence interval 76.85% to 91.84%) v 86.40% (79.21% to 91.37%). The sensitivity of HPV screening for CIN3+after five years was 89.34% (80.10% to 94.58%) and for cytology after three years was 92.02% (80.59% to 96.97%).
Conclusions: Over long term follow-up, the cumulative incidence of CIN2+ was the same for HPV screening and for cytology, implying that the increased sensitivity of HPV screening for CIN2+ reflects earlier detection rather than overdiagnosis. The low long term risks of CIN3+ among women who tested negative in HPV screening, support screening intervals of five years for such women.
Trial registration: Clinicaltrials.gov NCT00479375.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
More education is needed before we burden women with their HPV status.BMJ. 2014 Feb 12;348:g1443. doi: 10.1136/bmj.g1443. BMJ. 2014. PMID: 24523378 No abstract available.
-
The difference in sensitivity between HPV testing and cytology for detecting current and future CIN2+ increases over time.Evid Based Med. 2014 Oct;19(5):184. doi: 10.1136/eb-2014-101757. Epub 2014 May 1. Evid Based Med. 2014. PMID: 24785468 No abstract available.
References
-
- Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine 2012;30(Suppl 5):F12-23. - PubMed
-
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12-9. - PubMed
-
- Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 2012;30(Suppl 5):F88-99. - PubMed
-
- Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007;357:1589-97. - PubMed
-
- Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, et al. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst 2008;100:492-501. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
