A tRNA splicing operon: Archease endows RtcB with dual GTP/ATP cofactor specificity and accelerates RNA ligation
- PMID: 24435797
- PMCID: PMC3973293
- DOI: 10.1093/nar/gkt1375
A tRNA splicing operon: Archease endows RtcB with dual GTP/ATP cofactor specificity and accelerates RNA ligation
Abstract
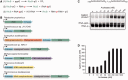
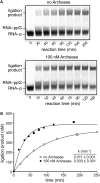
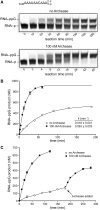
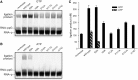
Archease is a 16-kDa protein that is conserved in all three domains of life. In diverse bacteria and archaea, the genes encoding Archease and the tRNA ligase RtcB are localized into an operon. Here we provide a rationale for this operon organization by showing that Archease and RtcB from Pyrococcus horikoshii function in tandem, with Archease altering the catalytic properties of the RNA ligase. RtcB catalyzes the GTP and Mn(II)-dependent joining of either 2',3'-cyclic phosphate or 3'-phosphate termini to 5'-hydroxyl termini. We find that catalytic concentrations of Archease are sufficient to activate RtcB, and that Archease accelerates both the RNA 3'-P guanylylation and ligation steps. In addition, we show that Archease can alter the NTP specificity of RtcB such that ATP, dGTP or ITP is used efficiently. Moreover, RtcB variants that have inactivating substitutions in the guanine-binding pocket can be rescued by the addition of Archease. We also present a 1.4 Å-resolution crystal structure of P. horikoshii Archease that reveals a metal-binding site consisting of conserved carboxylates located at the protein tip. Substitution of the Archease metal-binding residues drastically reduced Archease-dependent activation of RtcB. Thus, evolution has sought to co-express archease and rtcB by creating a tRNA splicing operon.
Figures


Similar articles
-
Coevolution of RtcB and Archease created a multiple-turnover RNA ligase.RNA. 2015 Nov;21(11):1866-72. doi: 10.1261/rna.052639.115. Epub 2015 Sep 18. RNA. 2015. PMID: 26385509 Free PMC article.
-
Structural and mechanistic insights into activation of the human RNA ligase RTCB by Archease.Nat Commun. 2024 Mar 16;15(1):2378. doi: 10.1038/s41467-024-46568-2. Nat Commun. 2024. PMID: 38493148 Free PMC article.
-
Structures of the noncanonical RNA ligase RtcB reveal the mechanism of histidine guanylylation.Biochemistry. 2013 Apr 16;52(15):2518-25. doi: 10.1021/bi4002375. Epub 2013 Apr 5. Biochemistry. 2013. PMID: 23560983 Free PMC article.
-
Insights into the structure and function of the RNA ligase RtcB.Cell Mol Life Sci. 2023 Nov 7;80(12):352. doi: 10.1007/s00018-023-05001-5. Cell Mol Life Sci. 2023. PMID: 37935993 Free PMC article. Review.
-
Structural basis for activation of an archaeal ribonuclease P RNA by protein cofactors.Biosci Biotechnol Biochem. 2017 Sep;81(9):1670-1680. doi: 10.1080/09168451.2017.1353404. Epub 2017 Jul 17. Biosci Biotechnol Biochem. 2017. PMID: 28715256 Review.
Cited by
-
RNA ligation in neurons by RtcB inhibits axon regeneration.Proc Natl Acad Sci U S A. 2015 Jul 7;112(27):8451-6. doi: 10.1073/pnas.1502948112. Epub 2015 Jun 22. Proc Natl Acad Sci U S A. 2015. PMID: 26100902 Free PMC article.
-
Large-Scale Molecular Evolutionary Analysis Uncovers a Variety of Polynucleotide Kinase Clp1 Family Proteins in the Three Domains of Life.Genome Biol Evol. 2019 Oct 1;11(10):2713-2726. doi: 10.1093/gbe/evz195. Genome Biol Evol. 2019. PMID: 31513263 Free PMC article.
-
The mammalian tRNA ligase complex mediates splicing of XBP1 mRNA and controls antibody secretion in plasma cells.EMBO J. 2014 Dec 17;33(24):2922-36. doi: 10.15252/embj.201490332. Epub 2014 Nov 6. EMBO J. 2014. PMID: 25378478 Free PMC article.
-
Regulation of Archease by the mTOR-vATPase axis.Development. 2022 Oct 1;149(19):dev200908. doi: 10.1242/dev.200908. Epub 2022 Oct 7. Development. 2022. PMID: 36111596 Free PMC article.
-
An unknown essential function of tRNA splicing endonuclease is linked to the integrated stress response and intron debranching.Genetics. 2023 May 26;224(2):iyad044. doi: 10.1093/genetics/iyad044. Genetics. 2023. PMID: 36943791 Free PMC article.
References
-
- Canaves JM. Predicted role for the Archease protein family based on structural and sequence analysis of TM1083 and MTH1598, two proteins structurally characterized through structural genomics efforts. Proteins. 2004;56:19–27. - PubMed
-
- Auxilien S, El Khadali F, Rasmussen A, Douthwaite S, Grosjean H. Archease from Pyrococcus abyssi improves substrate specificity and solubility of a tRNA m5C methyltransferase. J. Biol. Chem. 2007;282:18711–18721. - PubMed
-
- Martinez J. Keep digging and you will find: an exotic partner of the human tRNA ligase complex revealed by combining biochemistry and phyletic distribution. 2013 Abstract S006, Biogenesis and turnover of small RNAs. Royal Society, Edinburgh, UK, January 15th–17th.
-
- Popow J, Englert M, Weitzer S, Schleiffer A, Mierzwa B, Mechtler K, Trowitzsch S, Will CL, Lührmann R, Söll D, et al. HSPC117 is the essential subunit of a human tRNA splicing ligase complex. Science. 2011;331:760–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

