Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors
- PMID: 24436136
- PMCID: PMC3949157
- DOI: 10.1002/cncr.28434
Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors
Abstract
Background: The effective management of fatigue in patients with cancer requires a clear delineation of what constitutes nontrivial fatigue. The authors defined numeric cutpoints for fatigue severity based on functional interference and described the prevalence and characteristics of fatigue in patients with cancer and survivors.
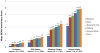
Methods: In a multicenter study, outpatients with breast, prostate, colorectal, or lung cancer rated their fatigue severity and symptom interference with functioning on the M. D. Anderson Symptom Inventory numeric scale of 0 to 10. Ratings of symptom interference guided the selection of numeric rating cutpoints between mild, moderate, and severe fatigue levels. Regression analysis identified significant factors related to reporting moderate=severe fatigue .
Results: The statistically optimal cutpoints were 4 for moderate fatigue and 7 for severe fatigue. Moderate=severe fatigue was reported by 983 of 2177 patients (45%) undergoing active treatment and was more likely to occur in patients receiving treatment with strong opioids (odds ratio [OR], 3.00), those with a poor Eastern Cooperative Oncology Group performance status (OR, 2.00), those who had >5% weight loss within 6 months (OR, 1.60), those who were receiving >10 medications (OR, 1.58), those with lung cancer (OR, 1.55), and those with a history of depression (OR, 1.42). Among survivors (patients with complete remission or no evidence of disease, and not currently receiving cancer treatment), 29% of patients (150 of 515 patients) had moderate=severe fatigue that was associated with poor performance status (OR, 3.48) and a history of depression (OR, 2.21).
Conclusions: The current study statistically defined fatigue severity categories related to significantly increased symptom interference. The high prevalence of moderate=severe fatigue in both actively treated patients with cancer and survivors warrants the promoting of the routine assessment and management of patient-reported fatigue.
Figures


References
-
- Yanez B, Pearman T, Lis CG, Beaumont JL, Cella D. The FACT-G7: a rapid version of the functional assessment of cancer therapy-general (FACT-G) for monitoring symptoms and concerns in oncology practice and research. Ann Oncol. 2013;24(4):1073–1078. - PubMed
-
- Minton O, Strasser F, Radbruch L, Stone P. Identification of factors associated with fatigue in advanced cancer: a subset analysis of the European palliative care research collaborative computerized symptom assessment data set. J Pain Symptom Manage. 2012;43(2):226–235. - PubMed
-
- Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. - PubMed
-
- Servaes P, Gielissen MF, Verhagen S, Bleijenberg G. The course of severe fatigue in disease-free breast cancer patients: a longitudinal study. Psychooncology. 2007;16(9):787–795. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

