Assessment of myeloperoxidase activity by the conversion of hydroethidine to 2-chloroethidium
- PMID: 24436331
- PMCID: PMC3937635
- DOI: 10.1074/jbc.M113.539486
Assessment of myeloperoxidase activity by the conversion of hydroethidine to 2-chloroethidium
Abstract
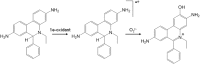
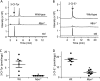
Oxidants derived from myeloperoxidase (MPO) contribute to inflammatory diseases. In vivo MPO activity is commonly assessed by the accumulation of 3-chlorotyrosine (3-Cl-Tyr), although 3-Cl-Tyr is formed at low yield and is subject to metabolism. Here we show that MPO activity can be assessed using hydroethidine (HE), a probe commonly employed for the detection of superoxide. Using LC/MS/MS, (1)H NMR, and two-dimensional NOESY, we identified 2-chloroethidium (2-Cl-E(+)) as a specific product when HE was exposed to hypochlorous acid (HOCl), chloramines, MPO/H2O2/chloride, and activated human neutrophils. The rate constant for HOCl-mediated conversion of HE to 2-Cl-E(+) was estimated to be 1.5 × 10(5) M(-1)s(-1). To investigate the utility of 2-Cl-E(+) to assess MPO activity in vivo, HE was injected into wild-type and MPO-deficient (Mpo(-/-)) mice with established peritonitis or localized arterial inflammation, and tissue levels of 2-Cl-E(+) and 3-Cl-Tyr were then determined by LC/MS/MS. In wild-type mice, 2-Cl-E(+) and 3-Cl-Tyr were detected readily in the peritonitis model, whereas in the arterial inflammation model 2-Cl-E(+) was present at comparatively lower concentrations (17 versus 0.3 pmol/mg of protein), and 3-Cl-Tyr could not be detected. Similar to the situation with 3-Cl-Tyr, tissue levels of 2-Cl-E(+) were decreased substantially in Mpo(-/-) mice, indicative of the specificity of the assay. In the arterial inflammation model, 2-Cl-E(+) was absent from non-inflamed arteries and blood, suggesting that HE oxidation occurred locally in the inflamed artery. Our data suggest that the conversion of exogenous HE to 2-Cl-E(+) may be a useful selective and sensitive marker for MPO activity in addition to 3-Cl-Tyr.
Keywords: 3-Chlorotyrosine; Chloramines; Dihydroethidium; HPLC; Hypochlorous Acid; Inflammation; Mass Spectrometry (MS); Neutrophil; Oxidative Stress.
Figures











References
-
- Halliwell B., Gutheridge J. M. C. (2007) Free Radicals in Biology and Medicine, 4th Ed., pp. 46–69, Oxford University Press, Oxford, UK
-
- Winterbourn C. C. (2008) Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 4, 278–286 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

