Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass
- PMID: 24437492
- PMCID: PMC3990840
- DOI: 10.1210/en.2013-1974
Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass
Abstract
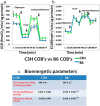
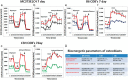
Osteoblastogenesis is the process by which mesenchymal stem cells differentiate into osteoblasts that synthesize collagen and mineralize matrix. The pace and magnitude of this process are determined by multiple genetic and environmental factors. Two inbred strains of mice, C3H/HeJ and C57BL/6J, exhibit differences in peak bone mass and bone formation. Although all the heritable factors that differ between these strains have not been elucidated, a recent F1 hybrid expression panel (C3H × B6) revealed major genotypic differences in osteoblastic genes related to cellular respiration and oxidative phosphorylation. Thus, we hypothesized that the metabolic rate of energy utilization by osteoblasts differed by strain and would ultimately contribute to differences in bone formation. In order to study the bioenergetic profile of osteoblasts, we measured oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) first in a preosteoblastic cell line MC3T3-E1C4 and subsequently in primary calvarial osteoblasts from C3H and B6 mice at days 7, 14, and 21 of differentiation. During osteoblast differentiation in media containing ascorbic acid and β-glycerophosphate, all 3 cell types increased their oxygen consumption and extracellular acidification rates compared with the same cells grown in regular media. These increases are sustained throughout differentiation. Importantly, C3H calvarial osteoblasts had greater oxygen consumption rates than B6 consistent with their in vivo phenotype of higher bone formation. Interestingly, osteoblasts utilized both oxidative phosphorylation and glycolysis during the differentiation process although mature osteoblasts were more dependent on glycolysis at the 21-day time point than oxidative phosphorylation. Thus, determinants of oxygen consumption reflect strain differences in bone mass and provide the first evidence that during collagen synthesis osteoblasts use both glycolysis and oxidative phosphorylation to synthesize and mineralize matrix.
Figures


References
-
- Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629–648 - PubMed
-
- Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18(5):397–403 - PubMed
-
- Zoidis E, Ghirlanda-Keller C, Schmid C. Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Mol Cell Biochem. 2011;348(1–2):33–42 - PubMed
-
- Thomas DM, Maher F, Rogers SD, Best JD. Expression and regulation by insulin of Glut 3 in UMR 106–01, a clonal rat osteosarcoma cell line. Biochem Biophys Res Commun. 1996;218(3):789–793 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

