Crystal structures of the human Dysferlin inner DysF domain
- PMID: 24438169
- PMCID: PMC3898210
- DOI: 10.1186/1472-6807-14-3
Crystal structures of the human Dysferlin inner DysF domain
Abstract
Background: Mutations in dysferlin, the first protein linked with the cell membrane repair mechanism, causes a group of muscular dystrophies called dysferlinopathies. Dysferlin is a type two-anchored membrane protein, with a single C terminal trans-membrane helix, and most of the protein lying in cytoplasm. Dysferlin contains several C2 domains and two DysF domains which are nested one inside the other. Many pathogenic point mutations fall in the DysF domain region.
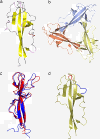
Results: We describe the crystal structure of the human dysferlin inner DysF domain with a resolution of 1.9 Ångstroms. Most of the pathogenic mutations are part of aromatic/arginine stacks that hold the domain in a folded conformation. The high resolution of the structure show that these interactions are a mixture of parallel ring/guanadinium stacking, perpendicular H bond stacking and aliphatic chain packing.
Conclusions: The high resolution structure of the Dysferlin DysF domain gives a template on which to interpret in detail the pathogenic mutations that lead to disease.
Figures


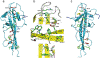
Similar articles
-
Pathogenic mutation R959W alters recognition dynamics of dysferlin inner DysF domain.Mol Biosyst. 2016 Mar;12(3):973-81. doi: 10.1039/c5mb00772k. Mol Biosyst. 2016. PMID: 26806107
-
Solution structure of the inner DysF domain of myoferlin and implications for limb girdle muscular dystrophy type 2b.J Mol Biol. 2008 Jun 20;379(5):981-90. doi: 10.1016/j.jmb.2008.04.046. Epub 2008 Apr 26. J Mol Biol. 2008. PMID: 18495154
-
Novel sequence variants in dysferlin-deficient muscular dystrophy leading to mRNA decay and possible C2-domain misfolding.Hum Mutat. 2006 Jun;27(6):599-600. doi: 10.1002/humu.9424. Hum Mutat. 2006. PMID: 16705711
-
Translational research and therapeutic perspectives in dysferlinopathies.Mol Med. 2011 Sep-Oct;17(9-10):875-82. doi: 10.2119/molmed.2011.00084. Epub 2011 May 6. Mol Med. 2011. PMID: 21556485 Free PMC article. Review.
-
Dysferlinopathies: Clinical and genetic variability.Clin Genet. 2022 Dec;102(6):465-473. doi: 10.1111/cge.14216. Epub 2022 Sep 6. Clin Genet. 2022. PMID: 36029111 Review.
Cited by
-
Structural Basis for the Distinct Membrane Binding Activity of the Homologous C2A Domains of Myoferlin and Dysferlin.J Mol Biol. 2019 May 17;431(11):2112-2126. doi: 10.1016/j.jmb.2019.04.006. Epub 2019 Apr 18. J Mol Biol. 2019. PMID: 31004665 Free PMC article.
-
Utilizing C. elegans Spermatogenesis and Fertilization Mutants as a Model for Human Disease.J Dev Biol. 2025 Jan 25;13(1):4. doi: 10.3390/jdb13010004. J Dev Biol. 2025. PMID: 39982357 Free PMC article. Review.
-
Role of calcium-sensor proteins in cell membrane repair.Biosci Rep. 2023 Feb 27;43(2):BSR20220765. doi: 10.1042/BSR20220765. Biosci Rep. 2023. PMID: 36728029 Free PMC article. Review.
-
Pex30-like proteins function as adaptors at distinct ER membrane contact sites.J Cell Biol. 2021 Oct 4;220(10):e202103176. doi: 10.1083/jcb.202103176. Epub 2021 Aug 17. J Cell Biol. 2021. PMID: 34402813 Free PMC article.
-
Miyoshi Muscular Dystrophy Type 1 with Mutated DYSF Gene Misdiagnosed as Becker Muscular Dystrophy: A Case Report and Literature Review.Genes (Basel). 2023 Jan 12;14(1):200. doi: 10.3390/genes14010200. Genes (Basel). 2023. PMID: 36672942 Free PMC article. Review.
References
-
- Illa I, Serrano-Munuera C, Gallardo E, Lasa A, Rojas-García R, Palmer J, Gallano P, Baiget M, Matsuda C, Brown RH. Distal anterior compartment myopathy: a dysferlin mutation causing a new muscular dystrophy phenotype. Ann Neurol. 2001;49:130–134. doi: 10.1002/1531-8249(200101)49:1<130::AID-ANA22>3.0.CO;2-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

