Comparison of two data collection processes in clinical studies: electronic and paper case report forms
- PMID: 24438227
- PMCID: PMC3909932
- DOI: 10.1186/1471-2288-14-7
Comparison of two data collection processes in clinical studies: electronic and paper case report forms
Abstract
Background: Electronic Case Report Forms (eCRFs) are increasingly chosen by investigators and sponsors of clinical research instead of the traditional pen-and-paper data collection (pCRFs). Previous studies suggested that eCRFs avoided mistakes, shortened the duration of clinical studies and reduced data collection costs.
Methods: Our objectives were to describe and contrast both objective and subjective efficiency of pCRF and eCRF use in clinical studies. A total of 27 studies (11 eCRF, 16 pCRF) sponsored by the Paris hospital consortium, conducted and completed between 2001 and 2011 were included. Questionnaires were emailed to investigators of those studies, as well as clinical research associates and data managers working in Paris hospitals, soliciting their level of satisfaction and preferences for eCRFs and pCRFs. Mean costs and timeframes were compared using bootstrap methods, linear and logistic regression.
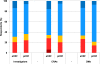
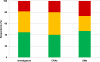
Results: The total cost per patient was 374€ ±351 with eCRFs vs. 1,135€ ±1,234 with pCRFs. Time between the opening of the first center and the database lock was 31.7 months Q1 = 24.6; Q3 = 42.8 using eCRFs, vs. 39.8 months Q1 = 31.7; Q3 = 52.2 with pCRFs (p = 0.11). Electronic CRFs were globally preferred by all (31/72 vs. 15/72 for paper) for easier monitoring and improved data quality.
Conclusions: This study found that eCRFs and pCRFs are used in studies with different patient numbers, center numbers and risk. The first ones are more advantageous in large, low-risk studies and gain support from a majority of stakeholders.
Figures


Similar articles
-
Data Quality and Cost-effectiveness Analyses of Electronic and Paper-Based Interviewer-Administered Public Health Surveys: Systematic Review.J Med Internet Res. 2021 Jan 22;23(1):e21382. doi: 10.2196/21382. J Med Internet Res. 2021. PMID: 33480859 Free PMC article.
-
Mobile electronic versus paper case report forms in clinical trials: a randomized controlled trial.BMC Med Res Methodol. 2017 Dec 1;17(1):153. doi: 10.1186/s12874-017-0429-y. BMC Med Res Methodol. 2017. PMID: 29191176 Free PMC article. Clinical Trial.
-
[Comparison of paper and electronic data management in clinical trials].Yao Xue Xue Bao. 2015 Nov;50(11):1461-3. Yao Xue Xue Bao. 2015. PMID: 26911043 Chinese.
-
Requirements for Evidence-Based Templates in Electronic Case Report Forms.Stud Health Technol Inform. 2016;223:142-9. Stud Health Technol Inform. 2016. PMID: 27139397
-
Limitations for health research with restricted data collection from UK primary care.Pharmacoepidemiol Drug Saf. 2019 Jun;28(6):777-787. doi: 10.1002/pds.4765. Epub 2019 Apr 16. Pharmacoepidemiol Drug Saf. 2019. PMID: 30993808 Free PMC article.
Cited by
-
Admissions to a Low-Resource Neonatal Unit in Malawi Using a Mobile App: Digital Perinatal Outcome Audit.JMIR Mhealth Uhealth. 2020 Oct 21;8(10):e16485. doi: 10.2196/16485. JMIR Mhealth Uhealth. 2020. PMID: 33084581 Free PMC article.
-
Improving the Efficacy of the Data Entry Process for Clinical Research With a Natural Language Processing-Driven Medical Information Extraction System: Quantitative Field Research.JMIR Med Inform. 2019 Jul 16;7(3):e13331. doi: 10.2196/13331. JMIR Med Inform. 2019. PMID: 31313661 Free PMC article.
-
Data Quality and Cost-effectiveness Analyses of Electronic and Paper-Based Interviewer-Administered Public Health Surveys: Systematic Review.J Med Internet Res. 2021 Jan 22;23(1):e21382. doi: 10.2196/21382. J Med Internet Res. 2021. PMID: 33480859 Free PMC article.
-
Evaluation of electronic patient-reported outcome assessment in inpatient cancer care: a feasibility study.Support Care Cancer. 2023 Sep 14;31(10):575. doi: 10.1007/s00520-023-08014-9. Support Care Cancer. 2023. PMID: 37707633 Free PMC article.
-
Integrating technology into complex intervention trial processes: a case study.Trials. 2016 Nov 17;17(1):551. doi: 10.1186/s13063-016-1674-9. Trials. 2016. PMID: 27855710 Free PMC article.
References
-
- Kuchinke W, Ohmann C, Yang Q, Salas N, Lauritsen J, Gueyffier F, Leizorovicz A, Schade-Brittinger C, Wittenberg M, Voko Z, Gaynor S, Cooney M, Doran P, Maggioni A, Lorimer A, Torres F, McPherson G, Charwill J, Hellstrom M, Lejeune S. Heterogeneity prevails: the state of clinical trial data management in Europe - results of a survey of ECRIN centres. Trials. 2010;11:79. doi: 10.1186/1745-6215-11-79. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

