Neuronal and astrocytic interactions modulate brain endothelial properties during metabolic stresses of in vitro cerebral ischemia
- PMID: 24438487
- PMCID: PMC3927849
- DOI: 10.1186/1478-811X-12-7
Neuronal and astrocytic interactions modulate brain endothelial properties during metabolic stresses of in vitro cerebral ischemia
Abstract
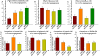
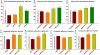
Neurovascular and gliovascular interactions significantly affect endothelial phenotype. Physiologically, brain endothelium attains several of its properties by its intimate association with neurons and astrocytes. However, during cerebrovascular pathologies such as cerebral ischemia, the uncoupling of neurovascular and gliovascular units can result in several phenotypical changes in brain endothelium. The role of neurovascular and gliovascular uncoupling in modulating brain endothelial properties during cerebral ischemia is not clear. Specifically, the roles of metabolic stresses involved in cerebral ischemia, including aglycemia, hypoxia and combined aglycemia and hypoxia (oxygen glucose deprivation and re-oxygenation, OGDR) in modulating neurovascular and gliovascular interactions are not known. The complex intimate interactions in neurovascular and gliovascular units are highly difficult to recapitulate in vitro. However, in the present study, we used a 3D co-culture model of brain endothelium with neurons and astrocytes in vitro reflecting an intimate neurovascular and gliovascular interactions in vivo. While the cellular signaling interactions in neurovascular and gliovascular units in vivo are much more complex than the 3D co-culture models in vitro, we were still able to observe several important phenotypical changes in brain endothelial properties by metabolically stressed neurons and astrocytes including changes in barrier, lymphocyte adhesive properties, endothelial cell adhesion molecule expression and in vitro angiogenic potential.
Figures
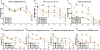
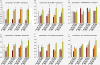
Similar articles
-
A potential gliovascular mechanism for microglial activation: differential phenotypic switching of microglia by endothelium versus astrocytes.J Neuroinflammation. 2018 May 15;15(1):143. doi: 10.1186/s12974-018-1189-2. J Neuroinflammation. 2018. PMID: 29764475 Free PMC article.
-
Gliovascular and cytokine interactions modulate brain endothelial barrier in vitro.J Neuroinflammation. 2011 Nov 23;8:162. doi: 10.1186/1742-2094-8-162. J Neuroinflammation. 2011. PMID: 22112345 Free PMC article.
-
Recovery of Neurovascular Unit Integrity by CDK5-KD Astrocyte Transplantation in a Global Cerebral Ischemia Model.Mol Neurobiol. 2018 Nov;55(11):8563-8585. doi: 10.1007/s12035-018-0992-1. Epub 2018 Mar 22. Mol Neurobiol. 2018. PMID: 29564811
-
Astrocyte-endothelial interactions at the blood-brain barrier.Nat Rev Neurosci. 2006 Jan;7(1):41-53. doi: 10.1038/nrn1824. Nat Rev Neurosci. 2006. PMID: 16371949 Review.
-
Targeting the neurovascular unit in brain trauma.CNS Neurosci Ther. 2015 Apr;21(4):304-8. doi: 10.1111/cns.12359. Epub 2014 Dec 5. CNS Neurosci Ther. 2015. PMID: 25475543 Free PMC article. Review.
Cited by
-
Inhibition of Complement Drives Increase in Early Growth Response Proteins and Neuroprotection Mediated by Salidroside After Cerebral Ischemia.Inflammation. 2018 Mar;41(2):449-463. doi: 10.1007/s10753-017-0701-7. Inflammation. 2018. PMID: 29198014
-
Limitations of Resting-State Functional MR Imaging in the Setting of Focal Brain Lesions.Neuroimaging Clin N Am. 2017 Nov;27(4):645-661. doi: 10.1016/j.nic.2017.06.004. Neuroimaging Clin N Am. 2017. PMID: 28985935 Free PMC article. Review.
-
Aging related impairment of brain microvascular bioenergetics involves oxidative phosphorylation and glycolytic pathways.J Cereb Blood Flow Metab. 2022 Aug;42(8):1410-1424. doi: 10.1177/0271678X211069266. Epub 2022 Mar 16. J Cereb Blood Flow Metab. 2022. PMID: 35296173 Free PMC article.
-
Promising Strategies for the Development of Advanced In Vitro Models with High Predictive Power in Ischaemic Stroke Research.Int J Mol Sci. 2022 Jun 27;23(13):7140. doi: 10.3390/ijms23137140. Int J Mol Sci. 2022. PMID: 35806146 Free PMC article. Review.
-
Value of Frequency Domain Resting-State Functional Magnetic Resonance Imaging Metrics Amplitude of Low-Frequency Fluctuation and Fractional Amplitude of Low-Frequency Fluctuation in the Assessment of Brain Tumor-Induced Neurovascular Uncoupling.Brain Connect. 2017 Aug;7(6):382-389. doi: 10.1089/brain.2016.0480. Brain Connect. 2017. PMID: 28657344 Free PMC article.
References
-
- Masamoto K, Tomita Y, Toriumi H, Aoki I, Unekawa M, Takuwa H. et al.Repeated longitudinal in vivo imaging of neuro-glio-vascular unit at the peripheral boundary of ischemia in mouse cerebral cortex. Neuroscience. 2012;212:190–200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

