New molecular medicine-based scar management strategies
- PMID: 24438742
- PMCID: PMC4008699
- DOI: 10.1016/j.burns.2013.11.010
New molecular medicine-based scar management strategies
Abstract
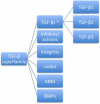
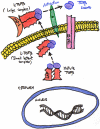
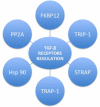
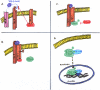
Keloids and hypertrophic scars are prevalent disabling conditions with still suboptimal treatments. Basic science and molecular-based medicine research have contributed to unravel new bench-to-bedside scar therapies and to dissect the complex signalling pathways involved. Peptides such as the transforming growth factor beta (TGF-β) superfamily, with Smads, Ski, SnoN, Fussels, endoglin, DS-Sily, Cav-1p, AZX100, thymosin-β4 and other related molecules may emerge as targets to prevent and treat keloids and hypertrophic scars. The aim of this review is to describe the basic complexity of these new molecular scar management strategies and point out new fibrosis research lines.
Keywords: Endoglin; FAP-alpha/DPPIV; Fussels; Keloid; Rapamycin; Review; Scar; Ski; SnoN; TGF-β.
Copyright © 2013 Elsevier Ltd and ISBI. All rights reserved.
Figures


References
-
- Mustoe TA, Cooter RD, Gold MH, Hobbs FD, Ramelet AA, Shakespeare PG, Stella M, Téot L, Wood FM, Ziegler UE. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110(2):560–71. - PubMed
-
- Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars. Arch Facial Plast Surg. 2006;8:362–8. - PubMed
-
- Rolfe KJ, Richardson J, Vigor C, Irvine LM, Grobbelaar AO, Linge C. A role for TGF-β1-induced cellular responses during wound healing of the non-scarring early human fetus ? J Invest Dermatol. 2007;127(11):2656–67. - PubMed
-
- Löhn P, Moren A, Raja E, Dahl M, Moustakas A. Regulating the stability of TGF-β receptors and Smads. Cell Res. 2009;19:21–35. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

