A blinded evaluation of the efficacy and safety of glycopyrronium, a once-daily long-acting muscarinic antagonist, versus tiotropium, in patients with COPD: the GLOW5 study
- PMID: 24438744
- PMCID: PMC3907130
- DOI: 10.1186/1471-2466-14-4
A blinded evaluation of the efficacy and safety of glycopyrronium, a once-daily long-acting muscarinic antagonist, versus tiotropium, in patients with COPD: the GLOW5 study
Abstract
Background: Two once-daily long-acting muscarinic antagonists (LAMAs) are currently available for the treatment of chronic obstructive pulmonary disease (COPD) - tiotropium and glycopyrronium. Previous studies have compared glycopyrronium with open-label tiotropium. In the GLOW5 study, we compare glycopyrronium with blinded tiotropium.
Methods: In this blinded, double-dummy, parallel group, 12-week study, patients with moderate-to-severe COPD were randomized 1:1 to glycopyrronium 50 μg once daily or tiotropium 18 μg once daily. The primary objective was to demonstrate the non-inferiority of glycopyrronium versus blinded tiotropium with respect to trough forced expiratory volume in 1 second (FEV1) following 12 weeks of treatment (non-inferiority margin: -50 mL). Secondary objectives were to evaluate glycopyrronium versus tiotropium for other spirometric outcomes, breathlessness (Transition Dyspnea Index; TDI), health status (St George's Respiratory Questionnaire; SGRQ), daily rescue medication use, COPD exacerbations and COPD symptoms over 12 weeks of treatment.
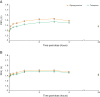
Results: 657 patients were randomized (glycopyrronium: 327; tiotropium: 330); 96% (630 patients) completed the study. Least squares mean trough FEV1 for both glycopyrronium and tiotropium was 1.405 L at Week 12, meeting the criterion for non-inferiority (mean treatment difference: 0 mL, 95% CI: -32, 31 mL). Glycopyrronium demonstrated rapid bronchodilation following first dose on Day 1, with significantly higher FEV1 at all time points from 0-4 h post-dose versus tiotropium (all p < 0.001). FEV1 area under the curve from 0-4 h (AUC0-4h) post-dose with glycopyrronium was significantly superior to tiotropium on Day 1 (p < 0.001) and was comparable to tiotropium at Week 12. Glycopyrronium demonstrated comparable improvements to tiotropium in TDI focal score, SGRQ total score, rescue medication use and the rate of COPD exacerbations (all p = not significant). Patients on glycopyrronium also had a significantly lower total COPD symptom score versus patients on tiotropium after 12 weeks (p = 0.035). Adverse events were reported by a similar percentage of patients receiving glycopyrronium (40.4%) and tiotropium (40.6%).
Conclusion: In patients with moderate-to-severe COPD, 12-week blinded treatment with once-daily glycopyrronium 50 μg or tiotropium 18 μg, provided similar efficacy and safety, with glycopyrronium having a faster onset of action on Day 1 versus tiotropium.
Trial registration: ClinicalTrials.gov NCT01613326.
Figures



References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2013. Global strategy for the diagnosis, management and prevention of COPD. 2013. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-managem.... - PubMed
-
- European Medicines Agency (EMA) Bretaris Genuair (aclidinium bromide) summary of product characteristics. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info....
-
- Food and Drug Administration (FDA) Tudorza pressair (aclidinium bromide) US prescribing information. 2013. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202450s000lbl.pdf.
-
- European Medicines Agency (EMA) Seebri Breezhaler (glycopyrronium bromide) Summary of Product Characteristics. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info....
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

