Adapting iron dose supplementation in pregnancy for greater effectiveness on mother and child health: protocol of the ECLIPSES randomized clinical trial
- PMID: 24438754
- PMCID: PMC3898489
- DOI: 10.1186/1471-2393-14-33
Adapting iron dose supplementation in pregnancy for greater effectiveness on mother and child health: protocol of the ECLIPSES randomized clinical trial
Abstract
Background: Currently, there is no consensus regarding iron supplementation dose that is most beneficial for maternal and offspring health during gestation. Recommended iron supplementation dose does not preempt anemia in around 20% of the pregnancies, nor the risk of hemoconcentration in 15%. This deficit, or excess, of iron prejudices the mother-child wellbeing. Therefore the aims of the study are to determine the highest level of effectiveness of iron supplementation adapted to hemoglobin (Hb) levels in early pregnancy, which would be optimum for mother-child health.
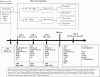
Design: Randomized Clinical Trial (RCT) triple-blindedSetting: 10 Primary Care Centers from Catalunya (Spain)Study subjects: 878 non-anemic pregnant women at early gestation stage, and their subsequent newborns
Methods: The study is structured as a RCT with 2 strata, depending on the Hb levels before week 12 of gestation. Stratum #1: If Hb from 110 to 130 g/L, randomly assigned at week 12 to receive iron supplement of 40 or 80 mg/d. Stratum #2: If Hb >130 g/L, randomly assigned at week 12 to receive iron supplement of 40 or 20 mg/d.
Measurements: In the mother: socio-economic data, clinical history, food item frequency, lifestyle and emotional state, and adherence to iron supplement prescription. Biochemical measurements include: Hb, serum ferritin, C reactive protein, cortisol, and alterations in the HFE gene (C282Y, H63D). In children: ultrasound fetal biometry, anthropometric measurements, and temperament development.Statistical analyses, using the SPSS program for Windows, will include bivariate and multivariate analyses adjusted for variables associated with the relationship under study.
Discussion: Should conclusive outcomes be reached, the study would indicate the optimal iron supplementation dose required to promote maternal and infant health. These results would contribute towards developing guidelines for good clinical practice.
References
-
- World Health Organization (WHO) Worldwide Prevalence of Anaemia 1993–2005. WHO Global Database on Anaemia. Geneva: WHO; 2008. whqlibdoc.who.int/publications/2008/9789241596657_eng.pdf.
-
- Peña-Rosas JP, Viteri FE. Effects and safety of preventive oral iron or iron + folic supplementation for women during pregnancy. Cochrane Database Syst Rev. 2009;4:CD004736. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials