The challenge and promise of glycomics
- PMID: 24439204
- PMCID: PMC3955176
- DOI: 10.1016/j.chembiol.2013.12.010
The challenge and promise of glycomics
Abstract
Glycomics is a broad and emerging scientific discipline focused on defining the structures and functional roles of glycans in biological systems. The staggering complexity of the glycome, minimally defined as the repertoire of glycans expressed in a cell or organism, has resulted in many challenges that must be overcome; these are being addressed by new advances in mass spectrometry as well as by the expansion of genetic and cell biology studies. Conversely, identifying the specific glycan recognition determinants of glycan-binding proteins by employing the new technology of glycan microarrays is providing insights into how glycans function in recognition and signaling within an organism and with microbes and pathogens. The promises of a more complete knowledge of glycomes are immense in that glycan modifications of intracellular and extracellular proteins have critical functions in almost all biological pathways.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
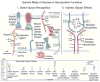


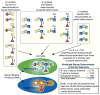
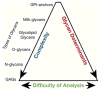


References
-
- Aebi M. N-linked protein glycosylation in the ER. Biochim Biophys Acta. 2013;1833:2430–2437. - PubMed
-
- Aoki K, Perlman M, Lim JM, Cantu R, Wells L, Tiemeyer M. Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J Biol Chem. 2007;282:9127–9142. - PubMed
-
- Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473:4–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 CA168930/CA/NCI NIH HHS/United States
- U01CA168930/CA/NCI NIH HHS/United States
- R01AI101982/AI/NIAID NIH HHS/United States
- P41 GM103490/GM/NIGMS NIH HHS/United States
- U01 CA128454/CA/NCI NIH HHS/United States
- P41 GM103694/GM/NIGMS NIH HHS/United States
- U01CA128454/CA/NCI NIH HHS/United States
- R24 GM098791/GM/NIGMS NIH HHS/United States
- P41GM103490/GM/NIGMS NIH HHS/United States
- R24GM098791/GM/NIGMS NIH HHS/United States
- P41GM103694/GM/NIGMS NIH HHS/United States
- R01 AI101982/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

