Chemical and chemoenzymatic synthesis of glycoproteins for deciphering functions
- PMID: 24439206
- PMCID: PMC3923302
- DOI: 10.1016/j.chembiol.2014.01.001
Chemical and chemoenzymatic synthesis of glycoproteins for deciphering functions
Abstract
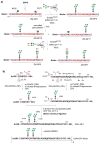
Glycoproteins are an important class of biomolecules involved in a number of biological recognition processes. However, natural and recombinant glycoproteins are usually produced as mixtures of glycoforms that differ in the structures of the pendent glycans, which are difficult to separate in pure glycoforms. As a result, synthetic homogeneous glycopeptides and glycoproteins have become indispensable probes for detailed structural and functional studies. A number of elegant chemical and biological strategies have been developed for synthetic construction of tailor-made, full-size glycoproteins to address specific biological problems. In this review, we highlight recent advances in chemical and chemoenzymatic synthesis of homogeneous glycoproteins. Selected examples are given to demonstrate the applications of tailor-made, glycan-defined glycoproteins for deciphering glycosylation functions.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures






Similar articles
-
Chemoenzymatic Methods for the Synthesis of Glycoproteins.Chem Rev. 2018 Sep 12;118(17):8359-8413. doi: 10.1021/acs.chemrev.8b00238. Epub 2018 Aug 24. Chem Rev. 2018. PMID: 30141327 Free PMC article. Review.
-
Expeditious chemoenzymatic synthesis of CD52 glycopeptide antigens.Org Biomol Chem. 2010 Nov 21;8(22):5224-33. doi: 10.1039/c0ob00341g. Epub 2010 Sep 17. Org Biomol Chem. 2010. PMID: 20848033 Free PMC article.
-
Carbohydrate synthesis and biosynthesis technologies for cracking of the glycan code: recent advances.Biotechnol Adv. 2013 Jan-Feb;31(1):17-37. doi: 10.1016/j.biotechadv.2012.03.008. Epub 2012 Mar 28. Biotechnol Adv. 2013. PMID: 22484115 Review.
-
Total synthesis of glycosylated proteins.Top Curr Chem. 2015;362:1-26. doi: 10.1007/128_2014_622. Top Curr Chem. 2015. PMID: 25805144 Free PMC article. Review.
-
Chemoenzymatic synthesis of glycopeptides and glycoproteins through endoglycosidase-catalyzed transglycosylation.Carbohydr Res. 2008 Jul 21;343(10-11):1509-22. doi: 10.1016/j.carres.2008.03.025. Epub 2008 Mar 27. Carbohydr Res. 2008. PMID: 18405887 Free PMC article. Review.
Cited by
-
Recent Advances in the Chemical Biology of N-Glycans.Molecules. 2021 Feb 16;26(4):1040. doi: 10.3390/molecules26041040. Molecules. 2021. PMID: 33669465 Free PMC article. Review.
-
Chemoenzymatic Defucosylation of Therapeutic Antibodies for Enhanced Effector Functions Using Bacterial α-Fucosidases.Methods Mol Biol. 2018;1827:367-380. doi: 10.1007/978-1-4939-8648-4_19. Methods Mol Biol. 2018. PMID: 30196507 Free PMC article.
-
Site-specific immobilization of endoglycosidases for streamlined chemoenzymatic glycan remodeling of antibodies.Carbohydr Res. 2018 Mar 22;458-459:77-84. doi: 10.1016/j.carres.2018.02.007. Epub 2018 Feb 15. Carbohydr Res. 2018. PMID: 29475193 Free PMC article.
-
Generation of a glycosylated asparagine residue through chemoselective acylation of a glycosylhydrazide.Carbohydr Res. 2020 Jul;493:108022. doi: 10.1016/j.carres.2020.108022. Epub 2020 May 4. Carbohydr Res. 2020. PMID: 32516600 Free PMC article.
-
Preparation of Complex Glycans From Natural Sources for Functional Study.Front Chem. 2020 Jul 3;8:508. doi: 10.3389/fchem.2020.00508. eCollection 2020. Front Chem. 2020. PMID: 32719769 Free PMC article. Review.
References
-
- Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. - PubMed
-
- Aebi M, Bernasconi R, Clerc S, Molinari M. N-glycan structures: recognition and processing in the ER. Trends Biochem Sci. 2010;35:74–82. - PubMed
-
- Aggarwal S. What’s fueling the biotech engine--2010 to 2011. Nat Biotechnol. 2011;29:1083–1089. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

