Neuronal control of metabolism through nutrient-dependent modulation of tracheal branching
- PMID: 24439370
- PMCID: PMC3898607
- DOI: 10.1016/j.cell.2013.12.008
Neuronal control of metabolism through nutrient-dependent modulation of tracheal branching
Abstract
During adaptive angiogenesis, a key process in the etiology and treatment of cancer and obesity, the vasculature changes to meet the metabolic needs of its target tissues. Although the cues governing vascular remodeling are not fully understood, target-derived signals are generally believed to underlie this process. Here, we identify an alternative mechanism by characterizing the previously unrecognized nutrient-dependent plasticity of the Drosophila tracheal system: a network of oxygen-delivering tubules developmentally akin to mammalian blood vessels. We find that this plasticity, particularly prominent in the intestine, drives--rather than responds to--metabolic change. Mechanistically, it is regulated by distinct populations of nutrient- and oxygen-responsive neurons that, through delivery of both local and systemic insulin- and VIP-like neuropeptides, sculpt the growth of specific tracheal subsets. Thus, we describe a novel mechanism by which nutritional cues modulate neuronal activity to give rise to organ-specific, long-lasting changes in vascular architecture.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



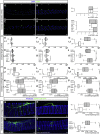

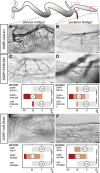

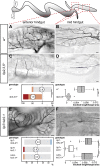
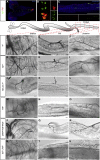




Comment in
-
Will branch for food-nutrient-dependent tracheal remodeling in Drosophila.EMBO J. 2014 Feb 3;33(3):179-80. doi: 10.1002/embj.201387412. Epub 2014 Jan 17. EMBO J. 2014. PMID: 24442634 Free PMC article.
References
-
- Adolphe J.L., Whiting S.J., Juurlink B.H., Thorpe L.U., Alcorn J. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br. J. Nutr. 2010;103:929–938. - PubMed
-
- Andersen D.S., Colombani J., Léopold P. Coordination of organ growth: principles and outstanding questions from the world of insects. Trends Cell Biol. 2013;23:336–344. - PubMed
-
- Belkacemi L., Nelson D.M., Desai M., Ross M.G. Maternal undernutrition influences placental-fetal development. Biol. Reprod. 2010;83:325–331. - PubMed
-
- Böhni R., Riesgo-Escovar J., Oldham S., Brogiolo W., Stocker H., Andruss B.F., Beckingham K., Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999;97:865–875. - PubMed
Supplemental References
-
- Allan D.W., St Pierre S.E., Miguel-Aliaga I., Thor S. Specification of neuropeptide cell identity by the integration of retrograde BMP signaling and a combinatorial transcription factor code. Cell. 2003;113:73–86. - PubMed
-
- Harrington D.P., Fleming T.R. A class of rank test procedures for censored survival data. Biometrika. 1982;69:553–566.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

