PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan
- PMID: 24439372
- PMCID: PMC3928474
- DOI: 10.1016/j.cell.2013.12.018
PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan
Abstract
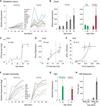
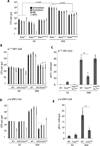
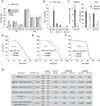
Interactions between commensals and the host impact the metabolic and immune status of metazoans. Their deregulation is associated with age-related pathologies like chronic inflammation and cancer, especially in barrier epithelia. Maintaining a healthy commensal population by preserving innate immune homeostasis in such epithelia thus promises to promote health and longevity. Here, we show that, in the aging intestine of Drosophila, chronic activation of the transcription factor Foxo reduces expression of peptidoglycan recognition protein SC2 (PGRP-SC2), a negative regulator of IMD/Relish innate immune signaling, and homolog of the anti-inflammatory molecules PGLYRP1-4. This repression causes deregulation of Rel/NFkB activity, resulting in commensal dysbiosis, stem cell hyperproliferation, and epithelial dysplasia. Restoring PGRP-SC2 expression in enterocytes of the intestinal epithelium, in turn, prevents dysbiosis, promotes tissue homeostasis, and extends lifespan. Our results highlight the importance of commensal control for lifespan of metazoans and identify SC-class PGRPs as longevity-promoting factors.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Tissue-Specific Regulation of Drosophila NF-x03BA;B Pathway Activation by Peptidoglycan Recognition Protein SC.J Innate Immun. 2016;8(1):67-80. doi: 10.1159/000437368. Epub 2015 Oct 30. J Innate Immun. 2016. PMID: 26513145 Free PMC article.
-
Drosophila as a model for intestinal dysbiosis and chronic inflammatory diseases.Dev Comp Immunol. 2014 Jan;42(1):102-10. doi: 10.1016/j.dci.2013.05.005. Epub 2013 May 16. Dev Comp Immunol. 2014. PMID: 23685204 Review.
-
Microbiota-Derived Lactate Activates Production of Reactive Oxygen Species by the Intestinal NADPH Oxidase Nox and Shortens Drosophila Lifespan.Immunity. 2018 Nov 20;49(5):929-942.e5. doi: 10.1016/j.immuni.2018.09.017. Epub 2018 Nov 13. Immunity. 2018. PMID: 30446385
-
Intestinal inflammation and stem cell homeostasis in aging Drosophila melanogaster.Front Cell Infect Microbiol. 2013 Dec 16;3:98. doi: 10.3389/fcimb.2013.00098. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 24380076 Free PMC article. Review.
-
The Drosophila peptidoglycan recognition protein PGRP-LF blocks PGRP-LC and IMD/JNK pathway activation.Cell Host Microbe. 2008 May 15;3(5):293-303. doi: 10.1016/j.chom.2008.04.002. Cell Host Microbe. 2008. PMID: 18474356
Cited by
-
Haemocytes control stem cell activity in the Drosophila intestine.Nat Cell Biol. 2015 Jun;17(6):736-48. doi: 10.1038/ncb3174. Epub 2015 May 25. Nat Cell Biol. 2015. PMID: 26005834 Free PMC article.
-
Bacteria-Derived Peptidoglycan Triggers a Noncanonical Nuclear Factor-κB-Dependent Response in Drosophila Gustatory Neurons.J Neurosci. 2022 Oct 12;42(41):7809-7823. doi: 10.1523/JNEUROSCI.2437-21.2022. Epub 2022 Sep 8. J Neurosci. 2022. PMID: 36414007 Free PMC article.
-
Intestine-derived α-synuclein initiates and aggravates pathogenesis of Parkinson's disease in Drosophila.Transl Neurodegener. 2022 Oct 17;11(1):44. doi: 10.1186/s40035-022-00318-w. Transl Neurodegener. 2022. PMID: 36253844 Free PMC article.
-
The Gut Microbiota and Healthy Aging: A Mini-Review.Gerontology. 2018;64(6):513-520. doi: 10.1159/000490615. Epub 2018 Jul 19. Gerontology. 2018. PMID: 30025401 Free PMC article. Review.
-
Control of Intestinal Cell Fate by Dynamic Mitotic Spindle Repositioning Influences Epithelial Homeostasis and Longevity.Cell Rep. 2019 Sep 10;28(11):2807-2823.e5. doi: 10.1016/j.celrep.2019.08.014. Cell Rep. 2019. PMID: 31509744 Free PMC article.
References
-
- Bakula M. The persistence of a microbial flora during postembryogenesis of Drosophila melanogaster. J Invertebr Pathol. 1969;14:365–374. - PubMed
-
- Becker T, Loch G, Beyer M, Zinke I, Aschenbrenner AC, Carrera P, Inhester T, Schultze JL, Hoch M. FOXO-dependent regulation of innate immune homeostasis. Nature. 2010;463:369–373. - PubMed
-
- Biagi E, Candela M, Turroni S, Garagnani P, Franceschi C, Brigidi P. Ageing and gut microbes: perspectives for health maintenance and longevity. Pharmacol Res. 2013;69:11–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

