Inefficient SRP interaction with a nascent chain triggers a mRNA quality control pathway
- PMID: 24439374
- PMCID: PMC3931426
- DOI: 10.1016/j.cell.2013.12.017
Inefficient SRP interaction with a nascent chain triggers a mRNA quality control pathway
Abstract
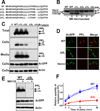
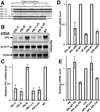
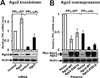
Misfolded proteins are often cytotoxic, unless cellular systems prevent their accumulation. Data presented here uncover a mechanism by which defects in secretory proteins lead to a dramatic reduction in their mRNAs and protein expression. When mutant signal sequences fail to bind to the signal recognition particle (SRP) at the ribosome exit site, the nascent chain instead contacts Argonaute2 (Ago2), and the mutant mRNAs are specifically degraded. Severity of signal sequence mutations correlated with increased proximity of Ago2 to nascent chain and mRNA degradation. Ago2 knockdown inhibited degradation of the mutant mRNA, while overexpression of Ago2 or knockdown of SRP54 promoted degradation of secretory protein mRNA. The results reveal a previously unappreciated general mechanism of translational quality control, in which specific mRNA degradation preemptively regulates aberrant protein production (RAPP).
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




References
-
- Albanese V, Yam AY, Baughman J, Parnot C, Frydman J. Systems analyses reveal two chaperone networks with distinct functions in eukaryotic cells. Cell. 2006;124:75–88. - PubMed
-
- Alder NN, Johnson AE. Cotranslational membrane protein biogenesis at the endoplasmic reticulum. J. Biol. Chem. 2004;279:22787–22790. - PubMed
-
- Ameyar-Zazoua M, Rachez C, Souidi M, Robin P, Fritsch L, Young R, Morozova N, Fenouil R, Descostes N, Andrau JC, et al. Argonaute proteins couple chromatin silencing to alternative splicing. Nat. Struct. Mol. Biol. 2012;19:998–1004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

