The WAVE regulatory complex links diverse receptors to the actin cytoskeleton
- PMID: 24439376
- PMCID: PMC4059610
- DOI: 10.1016/j.cell.2013.11.048
The WAVE regulatory complex links diverse receptors to the actin cytoskeleton
Abstract
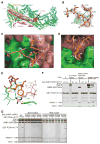
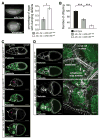
The WAVE regulatory complex (WRC) controls actin cytoskeletal dynamics throughout the cell by stimulating the actin-nucleating activity of the Arp2/3 complex at distinct membrane sites. However, the factors that recruit the WRC to specific locations remain poorly understood. Here, we have identified a large family of potential WRC ligands, consisting of ∼120 diverse membrane proteins, including protocadherins, ROBOs, netrin receptors, neuroligins, GPCRs, and channels. Structural, biochemical, and cellular studies reveal that a sequence motif that defines these ligands binds to a highly conserved interaction surface of the WRC formed by the Sra and Abi subunits. Mutating this binding surface in flies resulted in defects in actin cytoskeletal organization and egg morphology during oogenesis, leading to female sterility. Our findings directly link diverse membrane proteins to the WRC and actin cytoskeleton and have broad physiological and pathological ramifications in metazoans.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





Comment in
-
The shape of things to come.Cell. 2014 Jan 16;156(1-2):13-4. doi: 10.1016/j.cell.2013.12.037. Cell. 2014. PMID: 24439365
References
-
- Bastock R, St Johnston D. Drosophila oogenesis. Curr Biol. 2008;18:R1082–1087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

