Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue
- PMID: 24439382
- PMCID: PMC4862569
- DOI: 10.1016/j.cell.2013.11.044
Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue
Abstract
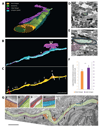
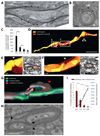
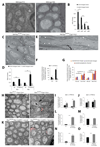
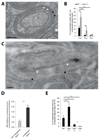
Central nervous system myelin is a multilayered membrane sheath generated by oligodendrocytes for rapid impulse propagation. However, the underlying mechanisms of myelin wrapping have remained unclear. Using an integrative approach of live imaging, electron microscopy, and genetics, we show that new myelin membranes are incorporated adjacent to the axon at the innermost tongue. Simultaneously, newly formed layers extend laterally, ultimately leading to the formation of a set of closely apposed paranodal loops. An elaborated system of cytoplasmic channels within the growing myelin sheath enables membrane trafficking to the leading edge. Most of these channels close with ongoing development but can be reopened in adults by experimentally raising phosphatidylinositol-(3,4,5)-triphosphate levels, which reinitiates myelin growth. Our model can explain assembly of myelin as a multilayered structure, abnormal myelin outfoldings in neurological disease, and plasticity of myelin biogenesis observed in adult life.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Myelin formation and remodeling.Cell. 2014 Jan 16;156(1-2):15-7. doi: 10.1016/j.cell.2013.12.038. Cell. 2014. PMID: 24439366 Free PMC article.
References
-
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. - PubMed
-
- Ben Geren B. The formation from the Schwann cell surface of myelin in the peripheral nerves of chick embryos. Exp Cell Res. 1954;7:558–562. - PubMed
-
- Blakemore WF. Schmidt-Lantermann incisures in the central nervous system. J Ultrastruct Res. 1969;29:496–498. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

