Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch
- PMID: 24439384
- PMCID: PMC3922400
- DOI: 10.1016/j.cell.2013.12.021
Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch
Abstract
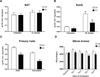
A clear relationship exists between visceral obesity and type 2 diabetes, whereas subcutaneous obesity is comparatively benign. Here, we show that adipocyte-specific deletion of the coregulatory protein PRDM16 caused minimal effects on classical brown fat but markedly inhibited beige adipocyte function in subcutaneous fat following cold exposure or β3-agonist treatment. These animals developed obesity on a high-fat diet, with severe insulin resistance and hepatic steatosis. They also showed altered fat distribution with markedly increased subcutaneous adiposity. Subcutaneous adipose tissue in mutant mice acquired many key properties of visceral fat, including decreased thermogenic and increased inflammatory gene expression and increased macrophage accumulation. Transplantation of subcutaneous fat into mice with diet-induced obesity showed a loss of metabolic benefit when tissues were derived from PRDM16 mutant animals. These findings indicate that PRDM16 and beige adipocytes are required for the "browning" of white fat and the healthful effects of subcutaneous adipose tissue.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
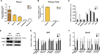





References
-
- Angulo P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002;346:1221–1231. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- P30 DK063720/DK/NIDDK NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- DK040936/DK/NIDDK NIH HHS/United States
- K08 DK097303/DK/NIDDK NIH HHS/United States
- R01 DK040936/DK/NIDDK NIH HHS/United States
- DK092541/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- R37 DK031405/DK/NIDDK NIH HHS/United States
- R01 DK031405/DK/NIDDK NIH HHS/United States
- DK045735/DK/NIDDK NIH HHS/United States
- DK031405/DK/NIDDK NIH HHS/United States
- R01 DK092541/DK/NIDDK NIH HHS/United States
- U24 DK059635/DK/NIDDK NIH HHS/United States
- DK059635/DK/NIDDK NIH HHS/United States
- P30 DK034989/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

