Measuring treatment response in psychotic depression: the Psychotic Depression Assessment Scale (PDAS) takes both depressive and psychotic symptoms into account
- PMID: 24439830
- PMCID: PMC3981944
- DOI: 10.1016/j.jad.2013.12.020
Measuring treatment response in psychotic depression: the Psychotic Depression Assessment Scale (PDAS) takes both depressive and psychotic symptoms into account
Abstract
Background: There is no established psychometric instrument dedicated to the measurement of severity in psychotic depression (PD). The aim of this study was to investigate whether a new composite rating scale, the Psychotic Depression Assessment Scale (PDAS), covering both the psychotic and the depressive domains of PD, could detect differences in effect between two psychopharmacological treatment regimens.
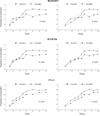
Methods: We reanalyzed the data from the Study of Pharmacotherapy of Psychotic Depression (STOP-PD), which compared the effect of Olanzapine+Sertraline (n=129) versus Olanzapine+Placebo (n=130). The response to the two regimens was compared using both a mixed effects model and effect size statistics on the total scores of three rating scales: the 17-item Hamilton Depression Rating Scale (HAM-D17), its 6-item melancholia subscale (HAM-D6), and the 11-item PDAS consisting of the HAM-D6 plus five items from the Brief Psychiatric Rating Scale covering psychotic symptoms.
Results: According to both statistical approaches, the PDAS, the HAM-D17 and the HAM-D6 were all able to detect significant differences in treatment effect between Olanzapine+Sertraline and Olanzapine+Placebo (Olanzapine+Sertraline being superior). Notably, 45% of the trial participants were at least "probable psychotic" at their last assessment in the trial.
Limitations: The STOP-PD was not designed specifically to answer the research questions of the present study.
Conclusions: The Psychotic Depression Assessment Scale (PDAS) is a sensitive measure of treatment response in PD. The fact that 45% of the patients still experienced psychotic symptoms at their last trial assessment emphasizes the need to include items pertaining to psychotic symptoms in rating scales for PD.
Keywords: Affective disorders, psychotic; Antidepressive agents; Antipsychotic agents; Psychiatric status rating scales.
Copyright © 2013 Elsevier B.V. All rights reserved.
Conflict of interest statement
S.D. Østergaard has received speaking fees, consultant honoraria and travel support from Janssen-Cilag until April 2011. Furthermore, he has received travel support at one occasion in 2010 from Bristol Myers Squibb. A.J. Rothschild has received grant support from the National Institute of Mental Health (NIMH), Cyberonics, Takeda, and St. Jude Medical and has served as a consultant to Allergan, GlaxoSmithKline, Eli Lilly, Noven Pharmaceuticals, Pfizer, Shire Pharmaceuticals, and Sunovian. A.J. Flint has received grant support from the NIMH, the Canadian Institutes of Health Research, and Lundbeck and has received honoraria from Janssen-Ortho, Lundbeck Canada, and Pfizer Canada. B.H. Mulsant currently receives research support from the Canadian Institutes of Health Research (CIHR), the US National Institute of Health (NIH), Bristol-Myers Squibb (medications for a NIH-funded clinical trial), and Pfizer (medications for a NIH-funded clinical trial). He directly own stocks of General Electric (less than $5,000). Within the past three years, he has also received some travel support from Roche. B. Meyers receives research support from the NIMH. He is receiving medication donated by Pfizer and Eli Lilly for his NIMH trial. During the last three years he has provided legal consultation to AstraZeneca and research consultation for Forest Laboratories. E.M. Whyte has received research support from the NIMH, the National Institute of Child Health and Human Development (NICHD), the Department of Defense (DOD) and through a Small Business Innovation Research (SBIR) grant from Fox Learning Systems / National Institute of Neurological Disorders and Stroke (NINDS). P. Bech and C. Ulbricht declare no conflicts of interest.
Figures
References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: 1994.
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Washington, DC: 2013.
-
- Angst J, Bech P, Boyer P, Bruinvels R, Engel R, Helmchen H, Hippius H, Lingjaerde O, Racagni G, Saletu B, Sedvall G, Silverstone JT, Stefanis CN, Stoll K, Woggon B. Consensus conference on the methodology of clinical trials of antidepressants, Zurich, march 1988: Report of the Consensus Committee. Pharmacopsychiatry. 1989;22:3–7.
-
- Bech P, Gram LF, Dein E, Jacobsen O, Vitger J, Bolwig TG. Quantitative rating of depressive states. Acta Psychiatr. Scand. 1975;51:161–170. - PubMed
-
- Bech P. Meta-analysis of placebo-controlled trials with mirtazapine using the core items of the Hamilton Depression Scale as evidence of a pure antidepressive effect in the short-term treatment of major depression. Int. J. Neuropsychopharmacol. 2001;4:337–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 MH062518/MH/NIMH NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- K24 MH069430/MH/NIMH NIH HHS/United States
- U01 MH062446/MH/NIMH NIH HHS/United States
- K23 MH067710/MH/NIMH NIH HHS/United States
- MH 62446/MH/NIMH NIH HHS/United States
- MH 62624/MH/NIMH NIH HHS/United States
- MH 62565/MH/NIMH NIH HHS/United States
- MH067710/MH/NIMH NIH HHS/United States
- M01-RR024153/RR/NCRR NIH HHS/United States
- UL1 TR000457/TR/NCATS NIH HHS/United States
- M01 RR000056/RR/NCRR NIH HHS/United States
- P30 MH068368/MH/NIMH NIH HHS/United States
- UL1 RR024996/RR/NCRR NIH HHS/United States
- MH 62518/MH/NIMH NIH HHS/United States
- UL1RR024996/RR/NCRR NIH HHS/United States
- RR000056/RR/NCRR NIH HHS/United States
- MH069430/MH/NIMH NIH HHS/United States
- U01 MH062624/MH/NIMH NIH HHS/United States
- U01 MH062565/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

