ABCC9 is a novel Brugada and early repolarization syndrome susceptibility gene
- PMID: 24439875
- PMCID: PMC3947869
- DOI: 10.1016/j.ijcard.2013.12.084
ABCC9 is a novel Brugada and early repolarization syndrome susceptibility gene
Abstract
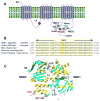
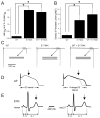
Background: Genetic defects in KCNJ8, encoding the Kir6.1 subunit of the ATP-sensitive K(+) channel (I(K-ATP)), have previously been associated with early repolarization (ERS) and Brugada (BrS) syndromes. Here we test the hypothesis that genetic variants in ABCC9, encoding the ATP-binding cassette transporter of IK-ATP (SUR2A), are also associated with both BrS and ERS.
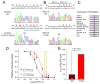
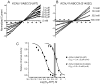
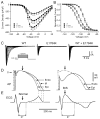
Methods and results: Direct sequencing of all ERS/BrS susceptibility genes was performed on 150 probands and family members. Whole-cell and inside-out patch-clamp methods were used to characterize mutant channels expressed in TSA201-cells. Eight ABCC9 mutations were uncovered in 11 male BrS probands. Four probands, diagnosed with ERS, carried a highly-conserved mutation, V734I-ABCC9. Functional expression of the V734I variant yielded a Mg-ATP IC₅₀ that was 5-fold that of wild-type (WT). An 18-y/o male with global ERS inherited an SCN5A-E1784K mutation from his mother, who displayed long QT intervals, and S1402C-ABCC9 mutation from his father, who displayed an ER pattern. ABCC9-S1402C likewise caused a gain of function of IK-ATP with a shift of ATP IC₅₀ from 8.5 ± 2 mM to 13.4 ± 5 μM (p<0.05). The SCN5A mutation reduced peak INa to 39% of WT (p<0.01), shifted steady-state inactivation by -18.0 mV (p<0.01) and increased late I(Na) from 0.14% to 2.01% of peak I(Na) (p<0.01).
Conclusion: Our study is the first to identify ABCC9 as a susceptibility gene for ERS and BrS. Our findings also suggest that a gain-of-function in I(K-ATP) when coupled with a loss-of-function in SCN5A may underlie type 3 ERS, which is associated with a severe arrhythmic phenotype.
Keywords: ATP-sensitive potassium channel; J wave syndromes; Mutation; Sodium channel; Sudden cardiac death.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Figures



References
-
- Mehta MC, Jain AC. Early repolarization on scalar electrocardiogram. Am J Med Sci. 1995;309:305–11. - PubMed
-
- Haissaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, De Roy L, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–23. - PubMed
-
- Rosso R, Kogan E, Belhassen B, Rozovski U, Scheinman MM, Zeltser D, et al. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J Am Coll Cardiol. 2008;52:1231–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

