Human gut microbes use multiple transporters to distinguish vitamin B₁₂ analogs and compete in the gut
- PMID: 24439897
- PMCID: PMC3923405
- DOI: 10.1016/j.chom.2013.12.007
Human gut microbes use multiple transporters to distinguish vitamin B₁₂ analogs and compete in the gut
Abstract
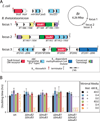
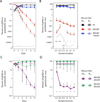
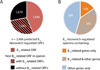
Genomic and metagenomic sequencing efforts, including human microbiome projects, reveal that microbes often encode multiple systems that appear to accomplish the same task. Whether these predictions reflect actual functional redundancies is unclear. We report that the prominent human gut symbiont Bacteroides thetaiotaomicron employs three functional, homologous vitamin B₁₂ transporters that in at least two cases confer a competitive advantage in the presence of distinct B₁₂ analogs (corrinoids). In the mammalian gut, microbial fitness can be determined by the presence or absence of a single transporter. The total number of distinct corrinoid transporter families in the human gut microbiome likely exceeds those observed in B. thetaiotaomicron by an order of magnitude. These results demonstrate that human gut microbes use elaborate mechanisms to capture and differentiate corrinoids in vivo and that apparent redundancies observed in these genomes can instead reflect hidden specificities that determine whether a microbe will colonize its host.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
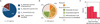



Comment in
-
Gut microbes take their vitamins.Cell Host Microbe. 2014 Jan 15;15(1):5-6. doi: 10.1016/j.chom.2013.12.011. Cell Host Microbe. 2014. PMID: 24439893 Free PMC article.
References
-
- Bookout AL, Cummins CL, Mangelsdorf DJ, Pesola JM, Kramer MF. High-throughput real-time quantitative reverse transcription PCR. Curr. Protoc. Mol. Biol. 2006;15:15.8.1–15.8.28. - PubMed
-
- Brandt LJ, Bernstein LH, Wagle A. Production of vitamin B12 analogues in patients with small-bowel bacterial overgrowth. Ann. Intern. Med. 1977;87:546–551. - PubMed
-
- Brown JR, Douady CJ, Italia MJ, Marshall WE, Stanhope MJ. Universal trees based on large combined protein sequence data sets. Nat. Genet. 2001;28:281–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

