Synergistic effects of amyloid-beta and wild-type human tau on dendritic spine loss in a floxed double transgenic model of Alzheimer's disease
- PMID: 24440055
- PMCID: PMC4072239
- DOI: 10.1016/j.nbd.2014.01.007
Synergistic effects of amyloid-beta and wild-type human tau on dendritic spine loss in a floxed double transgenic model of Alzheimer's disease
Abstract
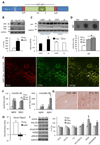
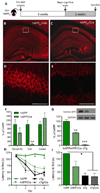
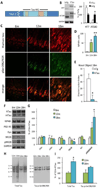
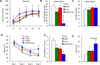
Synapse number is the best indicator of cognitive impairment In Alzheimer's disease (AD), yet the respective contributions of Aβ and tau, particularly human wild-type tau, to synapse loss remain undefined. Here, we sought to elucidate the Aβ-dependent changes in wild-type human tau that trigger synapse loss and cognitive decline in AD by generating two novel transgenic mouse models. The first overexpresses floxed human APP with Swedish and London mutations under the thy1 promoter, and recapitulates important features of early AD, including accumulation of soluble Aβ and oligomers, but no plaque formation. Transgene excision via Cre-recombinase reverses cognitive decline, even at 18-months of age. Secondly, we generated a human wild-type tau-overexpressing mouse. Crossing of the two animals accelerates cognitive impairment, causes enhanced accumulation and aggregation of tau, and results in reduction of dendritic spines compared to single transgenic hTau or hAPP mice. These results suggest that Aβ-dependent acceleration of wild-type human tau pathology is a critical component of the lasting changes to dendritic spines and cognitive impairment found in AD.
Keywords: Alzheimer's disease; Dendritic spines; Transgenic; Wild-type tau.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest: The authors declare no competing financial interests.
Figures

Similar articles
-
Human tau increases amyloid β plaque size but not amyloid β-mediated synapse loss in a novel mouse model of Alzheimer's disease.Eur J Neurosci. 2016 Dec;44(12):3056-3066. doi: 10.1111/ejn.13442. Epub 2016 Nov 12. Eur J Neurosci. 2016. PMID: 27748574 Free PMC article.
-
Neurofibrillary tangle formation by introducing wild-type human tau into APP transgenic mice.Acta Neuropathol. 2014 May;127(5):685-98. doi: 10.1007/s00401-014-1259-1. Epub 2014 Feb 15. Acta Neuropathol. 2014. PMID: 24531886
-
Familial Alzheimer's disease mutations at position 22 of the amyloid β-peptide sequence differentially affect synaptic loss, tau phosphorylation and neuronal cell death in an ex vivo system.PLoS One. 2020 Sep 23;15(9):e0239584. doi: 10.1371/journal.pone.0239584. eCollection 2020. PLoS One. 2020. PMID: 32966331 Free PMC article.
-
Spines, plasticity, and cognition in Alzheimer's model mice.Neural Plast. 2012;2012:319836. doi: 10.1155/2012/319836. Epub 2011 Nov 28. Neural Plast. 2012. PMID: 22203915 Free PMC article. Review.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
Cited by
-
Tau: The Center of a Signaling Nexus in Alzheimer's Disease.Front Neurosci. 2016 Feb 9;10:31. doi: 10.3389/fnins.2016.00031. eCollection 2016. Front Neurosci. 2016. PMID: 26903798 Free PMC article. Review.
-
Decreased nesting behavior, selective increases in locomotor activity in a novel environment, and paradoxically increased open arm exploration in Neurogranin knockout mice.Neuropsychopharmacol Rep. 2021 Mar;41(1):111-116. doi: 10.1002/npr2.12150. Epub 2020 Dec 3. Neuropsychopharmacol Rep. 2021. PMID: 33270377 Free PMC article.
-
β-amyloid accumulation enhances microtubule associated protein tau pathology in an APPNL-G-F/MAPTP301S mouse model of Alzheimer's disease.Front Neurosci. 2024 Mar 20;18:1372297. doi: 10.3389/fnins.2024.1372297. eCollection 2024. Front Neurosci. 2024. PMID: 38572146 Free PMC article.
-
Restoration of lipoxin A4 signaling reduces Alzheimer's disease-like pathology in the 3xTg-AD mouse model.J Alzheimers Dis. 2015;43(3):893-903. doi: 10.3233/JAD-141335. J Alzheimers Dis. 2015. PMID: 25125468 Free PMC article.
-
Amyloid-β: a potential link between epilepsy and cognitive decline.Nat Rev Neurol. 2021 Aug;17(8):469-485. doi: 10.1038/s41582-021-00505-9. Epub 2021 Jun 11. Nat Rev Neurol. 2021. PMID: 34117482 Review.
References
-
- Carlson GA, Borchelt DR, Dake A, Turner S, Danielson V, Coffin JD, Eckman C, Meiners J, Nilsen SP, Younkin SG, Hsiao KK. Genetic modification of the phenotypes produced by amyloid precursor protein overexpression in transgenic mice. Hum Mol Genet. 1997;6:1951–1959. - PubMed
-
- Caroni P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods. 1997;71:3–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

