A simple method for the determination of reduction potentials in heme proteins
- PMID: 24440354
- PMCID: PMC3999514
- DOI: 10.1016/j.febslet.2013.12.030
A simple method for the determination of reduction potentials in heme proteins
Abstract
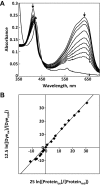
We describe a simple method for the determination of heme protein reduction potentials. We use the method to determine the reduction potentials for the PAS-A domains of the regulatory heme proteins human NPAS2 (Em=-115 mV ± 2 mV, pH 7.0) and human CLOCK (Em=-111 mV ± 2 mV, pH 7.0). We suggest that the method can be easily and routinely applied to the determination of reduction potentials across the family of heme proteins.
Keywords: Heme; Heme protein; Redox; Reduction potential.
Copyright © 2014 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Mauk A.G., Moore G.R. Control of metalloprotein redox potentials: what does site-directed mutagenesis of hemoproteins tell us? J. Biol. Inorg. Chem. 1997;2:119–125.
-
- Raven E.L., Mauk A.G. Chemical reactivity of the active site of myoglobin. Adv. Inorg. Chem. 2001;51:1–49.
-
- Battistuzzi G., Borsari M., Cowan J.A., Ranieri A., Sola M. Control of cytochrome C redox potential: axial ligation and protein environment effects. J. Am. Chem. Soc. 2002;124:5315–5324. - PubMed
-
- Pessanha M., Rothery E.L., Miles C.S., Reid G.A., Chapman S.K., Louro R.O., Turner D.L., Salgueiro C.A., Xavier A.V. Tuning of functional heme reduction potentials in Shewanella fumarate reductases. Biochim. Biophys. Acta. 2009;1787:113–120. - PubMed
-
- Bortolotti C.A., Amadei A., Aschi M., Borsari M., Corni S., Sola M., Daidone I. The reversible opening of water channels in cytochrome c modulates the heme iron reduction potential. J. Am. Chem. Soc. 2012;134:13670–13678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

