Neuronal over-expression of ACE2 protects brain from ischemia-induced damage
- PMID: 24440367
- PMCID: PMC3992949
- DOI: 10.1016/j.neuropharm.2014.01.004
Neuronal over-expression of ACE2 protects brain from ischemia-induced damage
Abstract
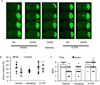
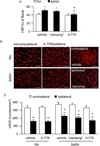
Angiotensin (Ang) II exaggerates cerebral injury in ischemic damage. Angiotensin-converting enzyme type 2 (ACE2) converts Ang II into Ang (1-7) and thus, may protect against the effects of Ang II. We hypothesized that neuronal ACE2 over-expression decreases ischemic stroke in mice with Ang II overproduction. Human renin and angiotensinogen double transgenic (RA) mice and RA mice with neuronal over-expression of ACE2 (SARA) were used for the study. The mean arterial pressure (MAP) was calculated from telemetry-recorded blood pressure (BP). SARA mice were infused peripherally with Norepinephrine to "clamp" the BP, or intracerebroventricularly-infused with a Mas receptor antagonist (A-779). Middle cerebral artery occlusion (MCAO) surgery was performed to induce permanent focal ischemic stroke. Cerebral blood flow (CBF) and neurological function were determined. Two days after surgery, brain samples were collected for various analyses. Results showed: 1) When compared to chronically hypertensive RA mice, SARA mice had lower basal MAP, less MCAO-induced infarct volume, and increased CBF, neurological function and cerebral microvascular density in the peri-infarct area; 2) These changes in SARA mice were not altered after MAP "clamping", but partially reversed by brain infusion of A-779; 3) Ang (1-7)/Ang II ratio, angiogenic factors, endothelial nitric oxide synthase (eNOS) expression and nitric oxide production were increased, whereas, NADPH oxidase subunits and reactive oxygen species were decreased in the brain of SARA mice. ACE2 protects brain from ischemic injury via the regulation of NADPH oxidase/eNOS pathways by changing Ang (1-7)/Ang II ratio, independently of MAP changes.
Keywords: Angiotensin II; Angiotensin-converting enzyme type 2; Blood pressure; Ischemic stroke; Oxidative stress.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures





References
-
- Beamer NB, Coull BM, Clark WM, Hazel JS, Silberger JR. Interleukin-6 and interleukin-1 receptor antagonist in acute stroke. Ann. Neurol. 1995;37:800–805. - PubMed
-
- Becker LK, Etelvino GM, Walther T, Santos RA, Campagnole-Santos MJ. Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am. J. Physiol Heart Circ. Physiol. 2007;293:H1416–H1424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

