Early steps in autophagy depend on direct phosphorylation of Atg9 by the Atg1 kinase
- PMID: 24440502
- PMCID: PMC3978657
- DOI: 10.1016/j.molcel.2013.12.011
Early steps in autophagy depend on direct phosphorylation of Atg9 by the Atg1 kinase
Erratum in
- Mol Cell. 2014 Feb 6;53(3):515
Abstract
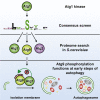
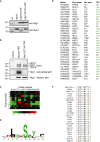
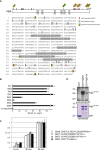
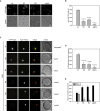
Bulk degradation of cytoplasmic material is mediated by a highly conserved intracellular trafficking pathway termed autophagy. This pathway is characterized by the formation of double-membrane vesicles termed autophagosomes engulfing the substrate and transporting it to the vacuole/lysosome for breakdown and recycling. The Atg1/ULK1 kinase is essential for this process; however, little is known about its targets and the means by which it controls autophagy. Here we have screened for Atg1 kinase substrates using consensus peptide arrays and identified three components of the autophagy machinery. The multimembrane-spanning protein Atg9 is a direct target of this kinase essential for autophagy. Phosphorylated Atg9 is then required for the efficient recruitment of Atg8 and Atg18 to the site of autophagosome formation and subsequent expansion of the isolation membrane, a prerequisite for a functioning autophagy pathway. These findings show that the Atg1 kinase acts early in autophagy by regulating the outgrowth of autophagosomal membranes.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Chen G.-C., Lee J.Y., Tang H.-W., Debnath J., Thomas S.M., Settleman J. Genetic interactions between Drosophila melanogaster Atg1 and paxillin reveal a role for paxillin in autophagosome formation. Autophagy. 2008;4:37–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

