Assessing PIK3CA and PTEN in early-phase trials with PI3K/AKT/mTOR inhibitors
- PMID: 24440717
- PMCID: PMC4409143
- DOI: 10.1016/j.celrep.2013.12.035
Assessing PIK3CA and PTEN in early-phase trials with PI3K/AKT/mTOR inhibitors
Abstract
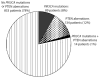
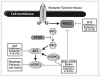
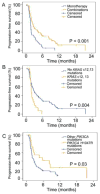
Despite a wealth of preclinical studies, it is unclear whether PIK3CA or phosphatase and tensin homolog (PTEN) gene aberrations are actionable in the clinical setting. Of 1,656 patients with advanced, refractory cancers tested for PIK3CA or PTEN abnormalities, PIK3CA mutations were found in 9% (146/1,589), and PTEN loss and/or mutation was found in 13% (149/1,157). In multicovariable analysis, treatment with a phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) inhibitor was the only independent factor predicting response to therapy in individuals harboring a PIK3CA or PTEN aberration. The rate of stable disease ≥6 months/partial response reached 45% in a subgroup of individuals with H1047R PIK3CA mutations. Aberrations in the PI3K/AKT/mTOR pathway are common and potentially actionable in patients with diverse advanced cancers. This work provides further important clinical validation for continued and accelerated use of biomarker-driven trials incorporating rational drug combinations.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Filip Janku has research support from Novartis. Razelle Kurzrock has research support from GlaxoSmithKline, Novartis, Merck, and Bayer.
Figures


References
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. - PubMed
-
- Cox D. Regression models and life tables (with discussion) J R Statistical Soc, B. 1972;34:187–220.
-
- De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

