UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model
- PMID: 24441054
- PMCID: PMC3902459
- DOI: 10.1136/bmjopen-2013-004073
UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model
Erratum in
- BMJ Open. 2014;4(1):e004073corr1. Zhu, Fheng [corrected to Zhu, Feng]
Abstract
Objectives: In 2002, the UK's National Institute for Health and Care Excellence concluded that the multiple sclerosis (MS) disease modifying therapies; interferon-β and glatiramer acetate, were not cost effective over the short term but recognised that reducing disability over the longer term might dramatically improve the cost effectiveness. The UK Risk-sharing Scheme (RSS) was established to ensure cost-effective provision by prospectively collecting disability-related data from UK-treated patients with MS and comparing findings to a natural history (untreated) cohort. However, deficiencies were found in the originally selected untreated cohort and the resulting analytical approach. This study aims to identify a more suitable natural history cohort and to develop a robust analytical approach using the new cohort.
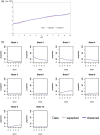
Design: The Scientific Advisory Group, recommended the British Columbia Multiple Sclerosis (BCMS) database, Canada, as providing a more suitable natural history comparator cohort. Transition probabilities were derived and different Markov models (discrete and continuous) with and without baseline covariates were applied.
Setting: MS clinics in Canada and the UK.
Participants: From the BCMS database, 898 'untreated' patients with MS considered eligible for drug treatment based on the UK's Association of British Neurologists criteria.
Outcome measure: The predicted Expanded Disability Status Scale (EDSS) score was collected and assessed for goodness of fit when compared with actual outcome.
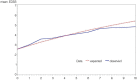
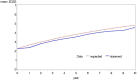
Results: The BCMS untreated cohort contributed 7335 EDSS scores over a median 6.4 years (6357 EDSS 'transitions' recorded at consecutive visits) during the period 1980-1995. A continuous Markov model with 'onset age' as a binary covariate was deemed the most suitable model for future RSS analysis.
Conclusions: A new untreated MS cohort from British Columbia has been selected and will be modelled using a continuous Markov model with onset age as a baseline covariate. This approach will now be applied to the treated UK RSS MS cohort for future price adjustment calculations.
Keywords: Markov model; glatiramer acetate; interferon-beta; quality of life; risk sharing scheme.
Figures
Similar articles
-
Effectiveness and cost-effectiveness of interferon beta and glatiramer acetate in the UK Multiple Sclerosis Risk Sharing Scheme at 6 years: a clinical cohort study with natural history comparator.Lancet Neurol. 2015 May;14(5):497-505. doi: 10.1016/S1474-4422(15)00018-6. Epub 2015 Apr 1. Lancet Neurol. 2015. PMID: 25841667
-
Modelling disease progression in relapsing-remitting onset multiple sclerosis using multilevel models applied to longitudinal data from two natural history cohorts and one treated cohort.Health Technol Assess. 2016 Oct;20(81):1-48. doi: 10.3310/hta20810. Health Technol Assess. 2016. PMID: 27817792 Free PMC article.
-
Assessing the long-term effectiveness of interferon-beta and glatiramer acetate in multiple sclerosis: final 10-year results from the UK multiple sclerosis risk-sharing scheme.J Neurol Neurosurg Psychiatry. 2019 Mar;90(3):251-260. doi: 10.1136/jnnp-2018-318360. Epub 2018 Sep 21. J Neurol Neurosurg Psychiatry. 2019. PMID: 30242090 Free PMC article.
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-beta and glatiramer acetate for multiple sclerosis.Health Technol Assess. 2004 Jun;8(27):iii, 1-78. doi: 10.3310/hta8270. Health Technol Assess. 2004. PMID: 15215017 Review.
Cited by
-
How patients with multiple sclerosis acquire disability.Brain. 2022 Sep 14;145(9):3147-3161. doi: 10.1093/brain/awac016. Brain. 2022. PMID: 35104840 Free PMC article.
-
Cladribine tablets are a cost-effective strategy in high-disease activity relapsing multiple sclerosis patients in Iran.Curr J Neurol. 2021 Jul 6;20(3):146-153. doi: 10.18502/cjn.v20i3.7690. Curr J Neurol. 2021. PMID: 38011415 Free PMC article.
-
Understanding Payer Perspectives on Value in the Use of Pharmaceuticals in the United States.J Manag Care Spec Pharm. 2019 Dec;25(12):1319-1327. doi: 10.18553/jmcp.2019.25.12.1319. J Manag Care Spec Pharm. 2019. PMID: 31778613 Free PMC article.
-
Long-term analysis of patients with benign multiple sclerosis: new insights about the disability course.J Neurol. 2021 Oct;268(10):3817-3825. doi: 10.1007/s00415-021-10501-0. Epub 2021 Mar 31. J Neurol. 2021. PMID: 33791847
-
Cost-consequence analysis of early vs. delayed natalizumab use in highly active relapsing-remitting multiple sclerosis: a simulation study.J Neurol. 2025 Jan 17;272(2):153. doi: 10.1007/s00415-024-12723-4. J Neurol. 2025. PMID: 39821478 Free PMC article.
References
-
- National Institute for Health and Care Excellence Beta interferon and glatiramer acetate for the treatment of multiple sclerosis. NICE Technology Appraisal Guidance No. 32. London: NICE, 2002
-
- Department of Health Cost effective provision of disease modifying therapies for people with multiple sclerosis. London: Health Service Circular 2002/004 London: Stationery Office, 2002
-
- Hemmett L, Holmes J, Barnes M, et al. What drives quality of life in multiple sclerosis? QJM 2004;97:671–6 - PubMed
-
- Kobelt G, Lindgren P, Parkin D, et al. Costs and quality of life in multiple sclerosis. A cross-sectional observational study in the UK. Scandinavian Working Papers in Economics, 2000. http://swopec.hhs.se/hastef/papers/hastef0398.pdf (accessed 28 Aug 2013)
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
